Calotropis procera L. (Asclepiadaceae) is a perennial shrub found in many areas of the Asian deserts (Gutter-man 1995). In these areas the average annual rainfall is between 30 and 200 mm, or less (Fu 1989). Calotropis sp. is distributed in Asia from the Mediterranean to the Africa coast. In Iran it is mainly distributed in the Fars province (Lamerd) and can be found in the Zahedan province as well. Iran is a country in the mid-latitude belt of arid and semi-arid regions of the Earth. Approximately 60% of Iran is classified as arid and semi-arid (Milton 1995). The main role of this species is in restoration of degraded lands and sustenance of these areas. In arid and semi arid lands, evaporation increases and rainfall is almost nonexistent during summer. As a consequence, the salt concentra-tion in topsoil increases (Lin and Chen 1995). Calotropis sp. regenerate by seed. Calotropis sp. produces lots of small seeds that dispread by wind. Establishment of the species in a new site depends on seed dispersal, germina-tion, and establishment of seedlings. Seed germination is extremely sensitive to soil salinity. Salinity stress reduces the percentage of germination and delays the initiation of the germination process (Baskin and Baskin 1997). Seeds of most halophytes germinate in non-saline conditions (Ungar 1995). Therefore, in saline conditions germination occurs after precipitation, where topsoil salinity is usually reduced (Heydecker et al. 1973). Ghaedi et al. (2010) re-ported that seeds of Haloxylon aphyllum L. as a halophyte is sensitive to salinity. Therefore, distribution of halo-phytes in saline environment depends on their germi-nation tolerance to salinity. Jhala (1997) recognized that there is a relation between salt tolerances at germination and the tolerance level during seedlings growth. There is little information available on the germination response on Calotropis procera L. seeds. Priming with a salinity stress had specific effects on different species (Gutterman 1997, Alimentacion et al. 2006, Khaef et al. 2011). In some species, seeds germinate when they are transferred from NaCl to distilled water (Khan and Rizvi 1994). In some halophyte like seeds of Medicago ruthenica and Salsola vermiculata, germination starts in distilled water after pretreatment under a saline or osmotic concentration (Alimentacion et al. 2006, Guan et al. 2009, Ghaedi et al. 2009). Calotropis sp. is an important economic plant used for drug and other purposes. Scientists wanted to under-stand its biological and ecological germination charac-teristics. Calotropis sp. regenerate by seeds and produce many seeds, but its density is very low in salty lands. It seems that germination of Calotropis sp. seeds is sensi-tive to salinity in the arid regions and occurs after rainfall when the soil salinity is reduced (Gulzar and Khan 2001). This study was conducted to investigate the effects of sa-linity on germination, seedling growth, and after priming of Calotropis procera L. seeds.
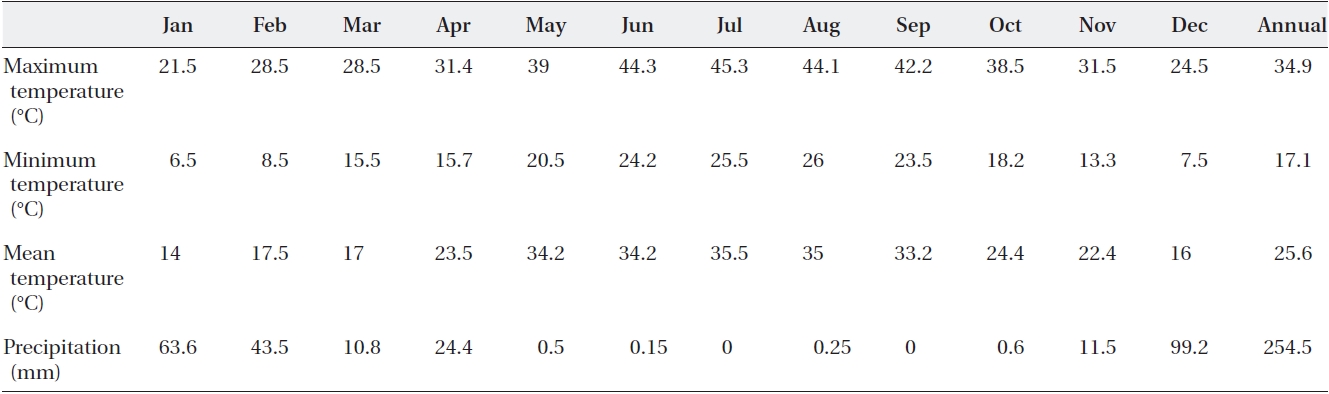
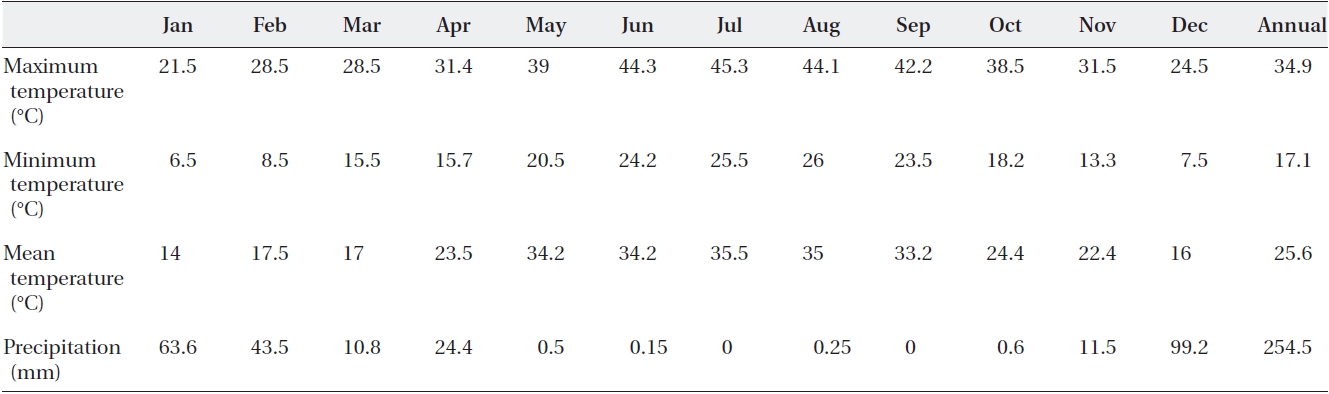
Mature seeds of Calotropis procera L. were collected from the shrubs of natural populations in the desert area in Fars province (Lamerd, 28° N and 53° E altitude) in 2010. The monthly precipitation and mean monthly tempera-tures are shown in Table 1. Seeds were hand separated from the pods and stored in a refrigerator at a temperature of 5℃. The experimental design was a complete random-ized block design with five levels of NaCl and CaCl2 iso-
bar concentrations: 0.0, -0.01, -0.05, -0.1, and -0.15 MPa. Experiments were carried out with four replicates of 50 seeds each, on Whatman No. 1 filter paper, in 90 mm di-ameter Petri dishes at 30℃. Seed germination was carried out under visible light of 400-700 nm wavelength. The seeds were considered to have been germinated when the lengths of the emerging radicals were over 2 mm (Interna-tional Seed Testing Association 1999). Germinated seed-lings were counted every day, for 15 days.
Calotropis procera L. seeds were treated with NaCl and CaCl2 (-0.1 MPa) at 30℃ for 4 days. The treated seeds were then transferred to levels of salinity (NaCl/CaCl2 ratio) po-tentials 0.0, -0.01, -0.05, -0.1, and -0.15 MPa. At the end of the experiment, the number of germinated seeds were observed and the percentage of germination was calcu-lated. Mean time to full germination (MTG) was calculat-ed according to the Eliss and Roberts equation (1981). The germination rate was calculated by inverse of MTG (Tobe et al. 2000) as follows:
MTG = Σ (ni.ti)/Σ n
GR = 1/MTG
MTG: Mean time to full germination
GR: germination rate
n: number of seeds newly germinating at time ‘t’
ti: number of day from sowing.
Seedling dry weights were measured after drying for 24 h in an oven at 70℃ (International Seed Testing Associa-tion 1999).
Data were analyzed using ‘MSTATC’ statistical software (MStatC Inc., East Lansing, MI, USA). Means were sepa-rated by Duncan test in cases in which the F-value of the treatments was significant at the P < 0.05 or P < 0.01 Prob-ability levels.
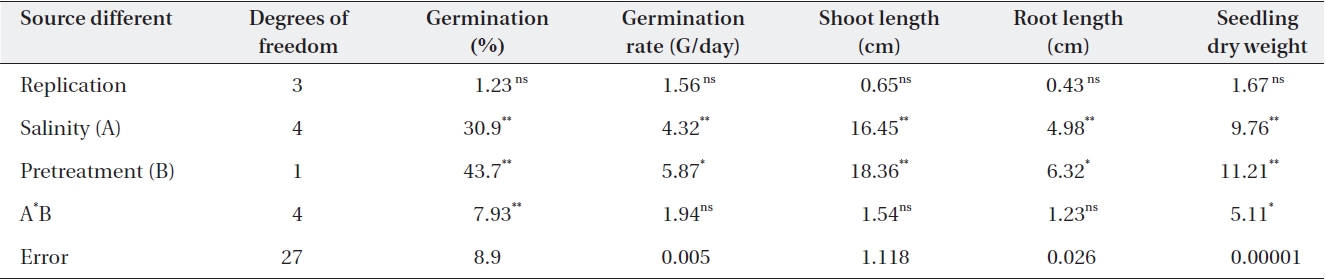
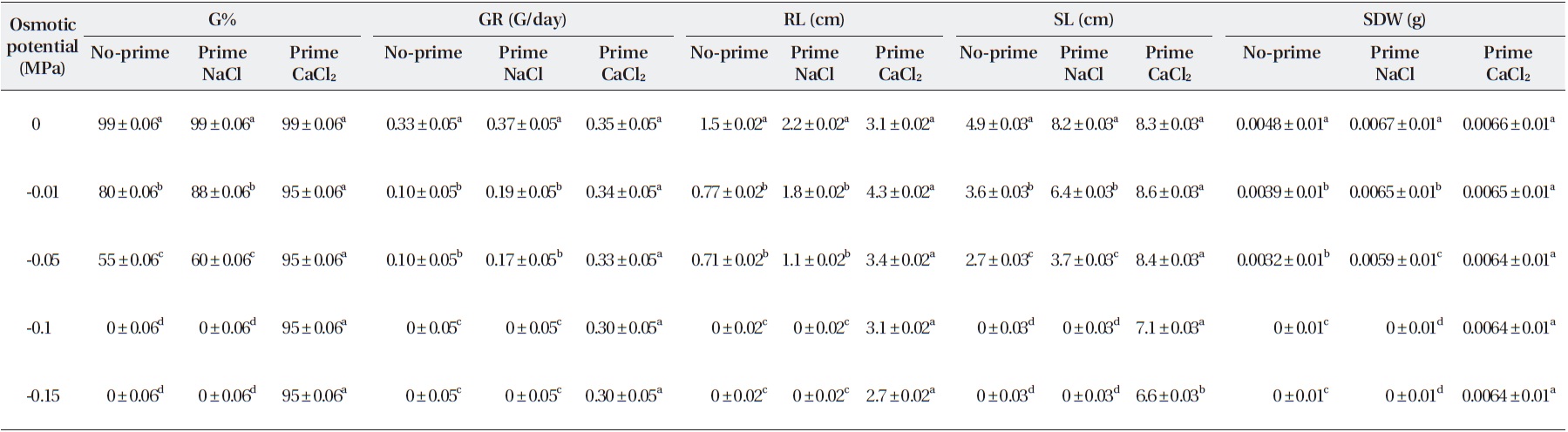
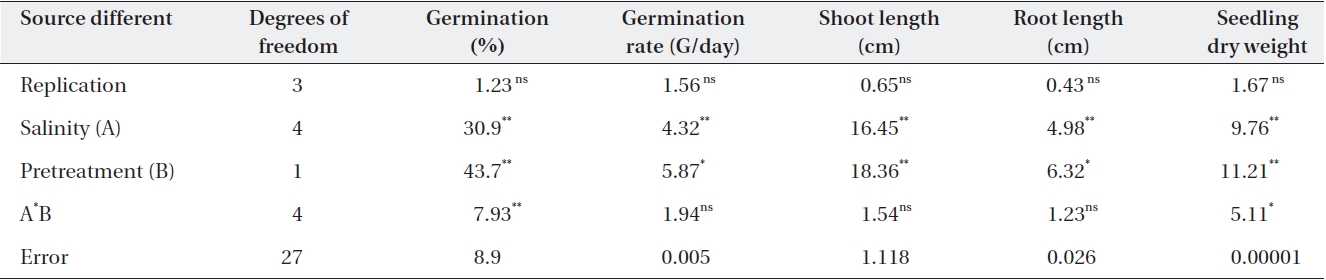
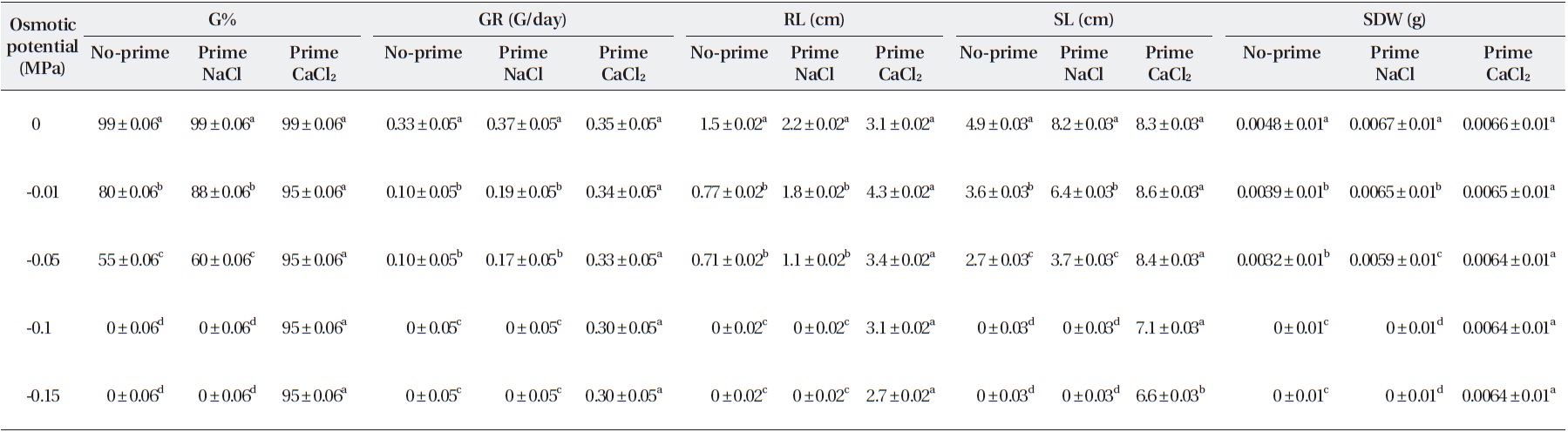
Salinity had significant effects on germination percent-ages (Table 2). The highest germination percentage was found in distilled water. The lowest germination percent-age was found in -0.05 MPa. Percentages of seed germina-tion at -0.01 MPa and -0.05 MPa were significantly differ-ent (Table 3). Priming by NaCl and CaCl2 had significant effects on germination percentages (Table 3). For example over all final germination percentages were comparable in both non-primed and primed seeds in the case of control and treatment with -0.1 MPa, but germination percent-age in primed seed with NaCl and CaCl2 were significantly higher than non-primed seeds. Germination occurred in -0.15 MPa in primed seeds with NaCl, but no germination was found in non-primed seeds (Table 3). Germination occurred in -0.15 MPa of CaCl2 prime (Table 3).
Germination rate was significantly affected by salinity (Table 2). Mean value showed that the highest germina-tion rate was found in distilled water (0.0 MPa). The low-est germination rate was found in -0.05 MP. Germina-tion rate at -0.01 and 0.0 MPa was significantly different (Table 2). Priming significantly improved the germina-tion rate (Table 3). The average germination rate in con-
trol for non-primed seeds with NaCl and CaCl2 was 0.33 (seed/h), while that of the primed seeds were 0.37 and 0.35 (seed/h), respectively. The germination rate of the primed seeds was significantly higher at over all levels of osmotic potentials of NaCl and CaCl2 than in non-primed seeds (Table 3).
Salinity had significant effects on root length (Table 2). The mean showed that the highest root length was found in distilled water. Root length in the control was 1.5 cm, but at -0.05 Mpa, it was only 0.71 and 3.2, respectively (Table 3). The lowest root length was found in -0.01 and -0.15 MPa of NaCl and CaCl2 (Table 3). Priming by NaCl and CaCl2 had significant effects on root length (Table 2). The priming increased the root length in all levels of os-motic potential studied (Table 3).
Salinity had significant effects on shoot length (Table 2). The mean showed that the highest shoot length was found in distilled water. The lowest shoot length was found in 0.01 and 0.05 MPa. Shoot length at 0.01 MPa and distilled water was significantly different (Table 3). Root length was significantly affected by priming with NaCl and CaCl2 in all levels of osmotic potential (Table 3). Root length in the control was 1.5 cm, but after priming with NaCl and CaCl2 increased to 8.2 and 8.3 cm, respec-tively, but decreased with decrease in osmotic potential (Table 2).
Seedling dry weights were significantly affected by sa-linity (Table 2). The mean showed that the highest seed-ling dry weight was found in distilled water. The lowest
seedling dry weight was found in 0.05 MPa. Seedling dry weights at 0.01 MPa and distilled water were significantly different (Table 2). Priming with NaCl had significant ef-fects on seedling dry weight (Table 2). The priming in-creased the seedling dry weight significantly (P < 0.01) in all levels of osmotic potential studied. Priming with NaCl increased the seedling dry weight more than that with CaCl2. However, there was no significant difference (Table 2).
The results showed that Calotropis sp. is sensitive to sa-linity in germination stage. Several perennial halophytes were sensitive to higher salinities during the germination phase (De Villiers et al. 1994, Delesalle and Blum 1994, Khan and Ungar 1998, Gulzar et al. 2001, Khan et al. 2001, Aiazzi et al. 2002). Germination and other characteristics of Calotropis seedlings decreased with increasing salin-ity, and were substantially inhibited at -0.1 and 0.15 MPa NaCl. Maximum germination was obtained in the non-saline and distilled water controls. Low osmotic potential decreases the water uptake of the seeds; thereby, inhibit-ing the germination (Dodd and Donovan 1999). Similar results were reported for Salsola vermiculata (Guma et al. 2010), Prosopis juliflora (El-Keblawy 2004), Medicago ru-thenica (Guan et al. 2009), Haloxylon aphyllum (Ghaedi et al. 2009), and a number of annual halophytes (Ungar 1995). Osmopriming had significant effects on germina-tion and characteristics of Calotropis sp. seedlings. Os-mopriming improved germination percentage, germina-tion rate, seedling length, and seedling dry weight of C. procera L. seeds (Table. 2 and 3). Similar findings were reported in Atriplex sp. (Katembe et al. 1998). For plants, sodium ions (Na+) are harmful, whereas potassium ions (K+) are essential. Under the typical NaCl-dominated salt environment in nature, accumulation of high Na+ in the cytosol, and thus high Na+/K+ ratios, disrupts the enzy-matic functions in cells (Munns et al. 2006). Therefore, it is very important for cells to maintain a low concentration of cytosolic Na+ or to maintain a low Na+/K+ ratio in the cytosol when under NaCl stress due to the toxic effects of high concentrations of Na+ (Maathuis and Amtmann 1999).
In general, the crops with the lowest Na+ concentra-tions produced greater dry matter. This low-Na+ genotype had fewer injured leaves, and a greater proportion of liv-ing to dead leaves (Munns and James 2003).
These results showed that in a natural environment, Calotropis seeds could remain in the soil of the field area when salinity levels were higher than their tolerance and after precipitation they normally grow (Ungar 1995). Ex-periments of prime germination identify that ion toxicity do not influence seeds of some halophytes (Salicornia eu-ropaea, Spergularia marina, Suaeda depressa, and Suaeda linearis), but osmotic effects reduce germination. Charac-teristics of H. recurvum were similar to that of Calotropis spin prime germination, since it is a halophyte and grows in the deserts of Pakistan (Khan and Ungar 1996). Priming is known as wetting under an osmotic potential or high salinity after seed washing. There are reports that priming permits early DNA replication (Bray et al. 1989), increased RNA and protein syntheses (Ibrahim et al. 1983), and re-sults in greater ATP availability (Mazor et al. 1984). Seeds with applied preconditioning or priming have higher lev-els of germination rates and uniformity in comparison with non-primed seeds (Heydecker et al. 1973, Osborne et al. 1981).
Deserts are a region where humidity is scarce because the potential of evapotranspiration is higher than that of precipitation. In certain areas of these regions, the water table is near the soil surface and due to the high rate of evaporation, salts accumulate on the soil surface (Khan and Ungar 1998). Finally, due to humidity, seedling roots begin to grow on the surface layer of the soil. Seedling sur-vival depends on the rapid growth of roots and their abil-ity to reach the moist layers of the soil. After precipitation the salinity of the soil is washed and reduced, and priming happens. Because of prime seeds that do not germinated in saline soil grow. Clotropis procera (Asclepiadaceae) is a perennial shrub that is not a halophyte. It can be con-cluded that this species may not be tolerant to extreme soil salinity during germination but is highly tolerant dur-ing storage in the soil. Recruitment from seeds would be facilitated whenever the window of opportunity is avail-able. Establishment of the species in a new site depends on the germination rate and establishment of seedlings. Seed germination in the plant life cycle is a critical stage for survival, especially under arid and unpredictable en-vironmental conditions like those of the Mediterranean ecosystems (Gimenez-Benavides et al. 2005). It seems that low distribution of Calotropis procera L. is sensitive to salinity at germination. But seeds of Calotropis proc-era L. are tolerant to salinity. Priming of Calotropis proc-era L. seeds increased tolerance to salinity, and increases the germination rate, root, and shoot lengths, number of leaves increases photosynthesis and seedling weight. This study provides some basic information related to the suit-ability of environmental conditions for better germina-tion of Calotropis procera L. seeds, which may be helpful.