Retention of coarse woody debris (CWD) is an important issue for sustainable forest management. CWD is any standing dead tree (snag), downed bole, or downed large branch (>10 cm in diameter) (Harmon et al. 1986, Spies et al. 1988, Yan et al. 2006). Many small mammals such as deer mice depend on CWD in forest ecosystems. Studies on small mammals in forests of the Pacific Northwest USA (Maser et al. 1979, Carey and Johnson 1995), southestern pine forests in the USA (Loeb 1999, Greenberg 2002), and Korean mixed forests (Kim et al. 2006) have indicated that the abundance of CWD is a good predictor of the small mammal abundance in forested ecosystems. Small mammals that live in any given habitat type depend on the differential use of microhabitats (Morrison and Anthony 1989, Carey and Harrington 2001). Additionally, CWD can provide for groups of taxa that are heavily dependent on moisture as well as temperature sensitive groups such as amphibians and reptiles (Owens et al. 2008). Small mammals use CWD as a necessary ground habitat feature, indicating that microhabitat is important with increasing population stability and reproductive benefits (Lee 1995). Small mammals including insectivores in the forest depend on CWD in the course of their life history, because CWD provides cover and forms survival and breeding microhabitats. Thus, microhabitat studies have clarified small mammal affinities for particular microsite features within a habitat.
The amount of CWD in old-growth forests is frequently sufficient for small mammal survival (West 1991, Ucitel et al. 2003) but may be a limiting factor in second-growth forests where CWD loads are lower (Lee 2004). Therefore, it would be instructive to examine the relationships between CWD and small mammals in managed forests, particularly considering that managed forests compromise > 80% of forests in the Pacific Northwest. The objectives of this study were to compare small mammal communities between sites with low and high densities of CWD in managed forests and to describe the associations between CWD and small mammal species. It was hypothesized that variations in CWD influence small mammal communities and microhabitat associations.
This study was conducted on the Ft. Lewis Military Reservation, east of the southern tip of Puget Trough Province in Pierce County, WA, USA. The study area was relatively flat with an average elevation of 250 m above sea level, and Douglas fir (
Three paired sites were selected; three with low densities of CWD (treatment) and three with high densities of CWD (control). Sites were paired based on relative proximity (two pairs with sites within 2 km of each other and one pair with sites within 5 km) to minimize the variation between the treatment and control. Low CWD sites had woody material, particularly logs, removed around 1910 to minimize forest fire hazards. Data were collected in 1991-1993 throughout the season with 2-week intervals for 3 days of each trapping period (refer to Fig. 2 in Lee 1995 for population dynamics).
Length, diameter at both ends, decay class (Sollins 1982), species, and location of downed logs were measured. The amount of CWD was also determined. Downed pieces ≥ 10 cm diameter at the small end were counted as downed logs. Circumference of the base and height of all snags and diameter and height of all stumps was measured. Some volumes of CWD (volume of logs, stumps, and snags) were measured following Lee (1995).
>
Animal capture using Sherman and Pitfall traps
Small mammal trapping was conducted on a 1-ha grid at each of the six sites. Each sampling grid consisted of a 10 × 10 array of stations with 10 m spacing. One Sherman collapsible trap (7.6 × 8.9 × 22.9 cm) was placed within a 2 m radius of each station. Each trap contained synthetic bedding and rolled oats for bait. The traps were baited and opened in late afternoon and checked and closed in the early morning. Each trap grid was normally run for 2 consecutive days twice per month for 25 months (June 1991-June 1993) except for November- February when trapping occurred once per month. Trapping was not conducted in January and February 1993 due to unusually cold conditions.
The sex, age (adult, subadult), weight, and reproductive condition of all trapped animals were individually recorded. Males were described as either scrotal (descended testes) or non-scrotal (abdominal testes). Females were described as either nonreproductive or reproductive (pregnant or lactating).
Twenty-five pitfall traps, spaced 10 m apart, were placed in a 5 × 5 grid within the center of a trapping grid. Pitfalls consisted of no. 10 tin cans (15 cm in diameter) buried flush with the surface of the soil and protected with a piece of wood propped one to several cm over the can lip. Pitfall traps were filled with water to a depth of 5-10 cm to reduce escapes and hasten mortality. Pitfalls were opened 2 consecutive nights every 2 weeks.
The abundance of each species in the community was analyzed using a paired
To determine the ecological relationship of deer mice to CWD, Poisson regression was selected to model seven response variables (total captures, captures of males, captures of females, proportion of males captured, captures of adults, captures of subadults, and captures by season [summer vs. winter]) as a function of CWD, pairs of sites, and low vs. high CWD sites (Zar 1999). Summer was defined as May-October and winter as November-April. Thirteen variables were used as predictor variables for CWD function (total volume of CWD, volume of logs, log volume multiplied by distance, volume of stumps, stump volume multiplied by distance, total number of CWD, number of logs, number of stumps, mean decay class, mean distance to capture point, volume of snag, and block, treatment). Block and treatment effects were considered macrohabitat factors in all models.
To determine the ecological relationship of CWD with insectivores, a Poisson regression was also selected to model the number of insectivore captures as a function of CWD, pairs of sites, and low vs. high CWD sites (Zar 1999). Five response variables were used in the analyses (total number of insectivore captures, captures of shrew-moles [
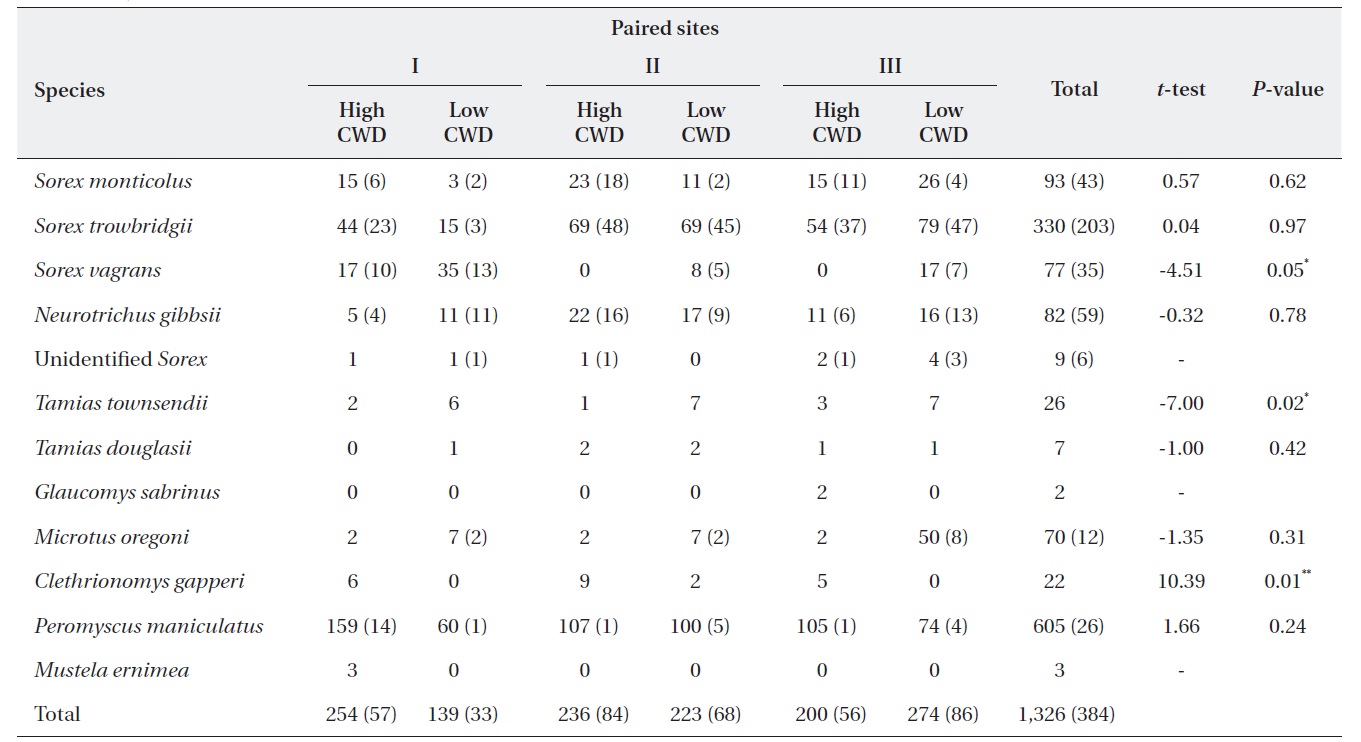
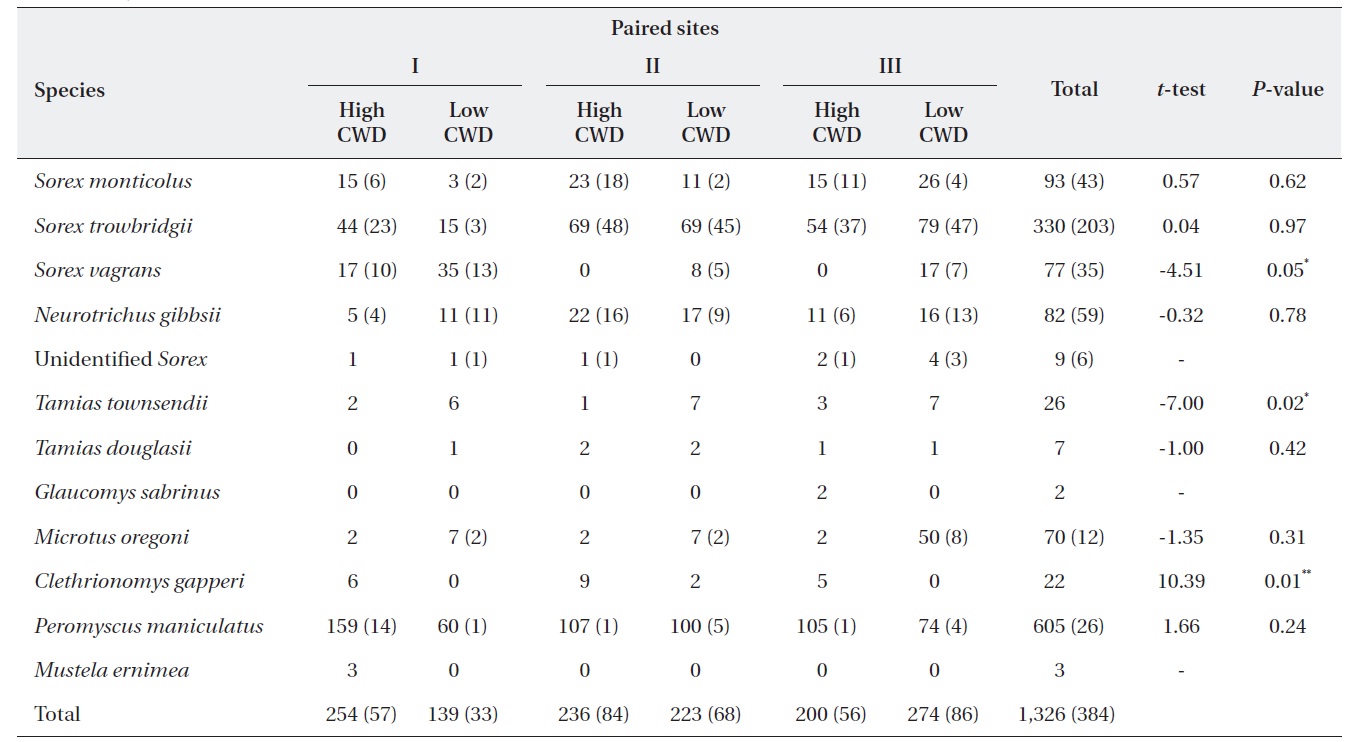
Total number of individual small mammals captured in both live and pitfall traps for each site and paired t-test results between high and low coarse woody debris (CWD) sites
The total volume of all CWD was higher (
>
Microhabitat analysis of deer mice and insectivores
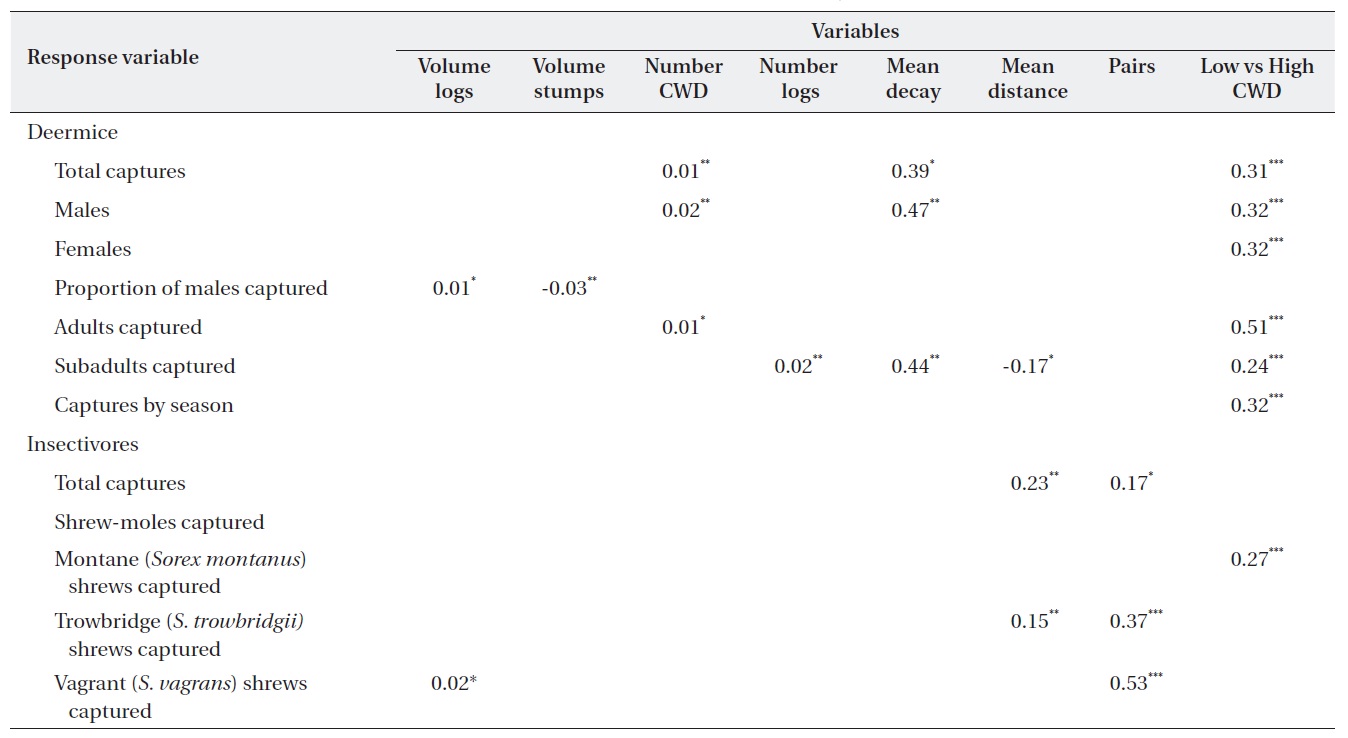
The regression models used 582 individual (380 Sherman traps and 242 pitfall) insectivores from Table 1. Data from 1,425 captured deer mice (data not shown) were used to assess microhabitat structure. All Poisson regression models indicated a significant difference between treatment and control sites; that is, treatment was always a significant variable (Table 2). Of the 13 microhabitat predictor variables, only six were related to microhabitat use of CWD (Table 2). The predictor variables of the total number of CWD pieces and mean decay were significant for predicting total captures of deer mice (Table 2). Total captures of males was significantly related to the number of CWD pieces and mean decay but captures of females were not significantly related to any of the 11 variables. The proportion of males captured was significantly related to both the volume of logs and stumps (Table 2). Microhabitat use by age class showed that total adult captures were significantly related only to CWD. In contrast, the number of logs, mean decay class, and mean distance from the capture points had a significant effect on subadult captures. The proportion of adults was 39.5%. Microhabitat use between summer and winter was not different (data not shown).
In the insectivore analyses, the Poisson model showed that only
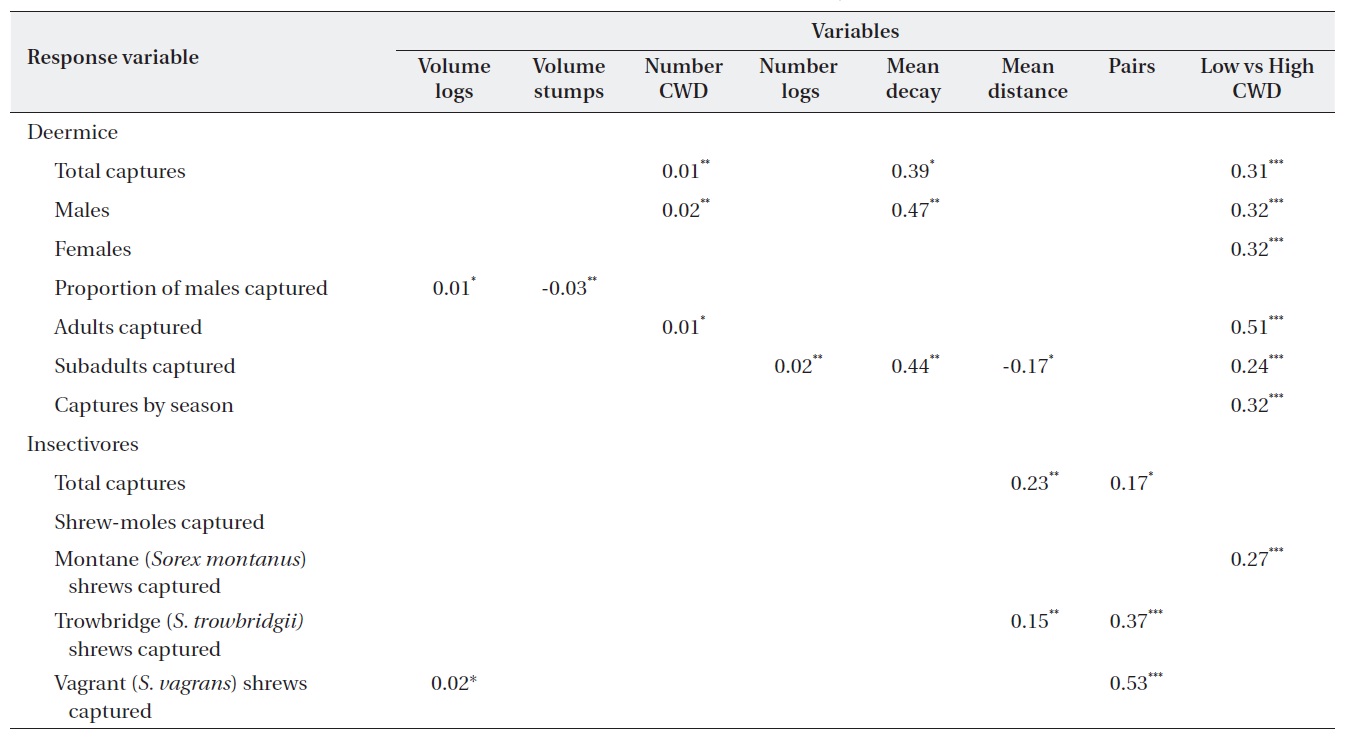
A list of significant coefficients from the generalized linear model for deer mice (Peromyscus maniculatus) and insectivores
The insectivorous community in this study was dominated by
Although the number of deer mice was not different between sites with low and high CWD, within-site use signified the importance of the CWD. Capture frequency of
We also found that subadult deer mice were closely associated with some characteristics of the CWD (number of logs, mean decay class, and mean distance). It seemed that the area covered by adults was larger than that of subadults, resulting in a better correlation between habitat characteristics of the subadult population and CWD.
Although a 5 m radius was used to estimate the total effect of CWD on insectivores, the mean distance from CWD to the capture station appeared to be the only significant variable for
Two conclusions can be reached: 1) the number of pieces of CWD and mean decay class, but not volume, were important for
In conclusion, the importance of CWD as a habitat feature for small mammals was tested at sites with high and low amounts of CWD. Analysis of within-site variability revealed that the number of pieces of CWD and mean decay class were positively related to deer mice captures. Captures of insectivores at trapping stations were highly associated with mean distance from CWD indicating that proximity to CWD was important to insectivores. Retention of large CWD can be an important for rodent management in forests of the Pacific Northwest.