Mile-a-minute (Mikania micrantha), a neo-tropical perennial vine, has become an important invasive plant species in many subtropical and tropical Asian countries including Nepal (Waterhouse 1994, Tiwari et al. 2005, Willis et al. 2008). This vine is listed as one of the world’s 32 worst invasive plants (Lowe et al. 2000), as it is notorious for its vigorous and rampant growth and rapid proliferation from both seed and vegetative parts (Kuo et al. 2002). Unlike other invasive plants, M. micrantha not only displaces native vegetation but also kills it. It climbs up to the top of the canopy and creates a dense cover that damages or kills other plants by blocking light (Holm et al. 1977). Hence, M. micrantha is competent to homogenize the invaded landscape and create a monoculture.
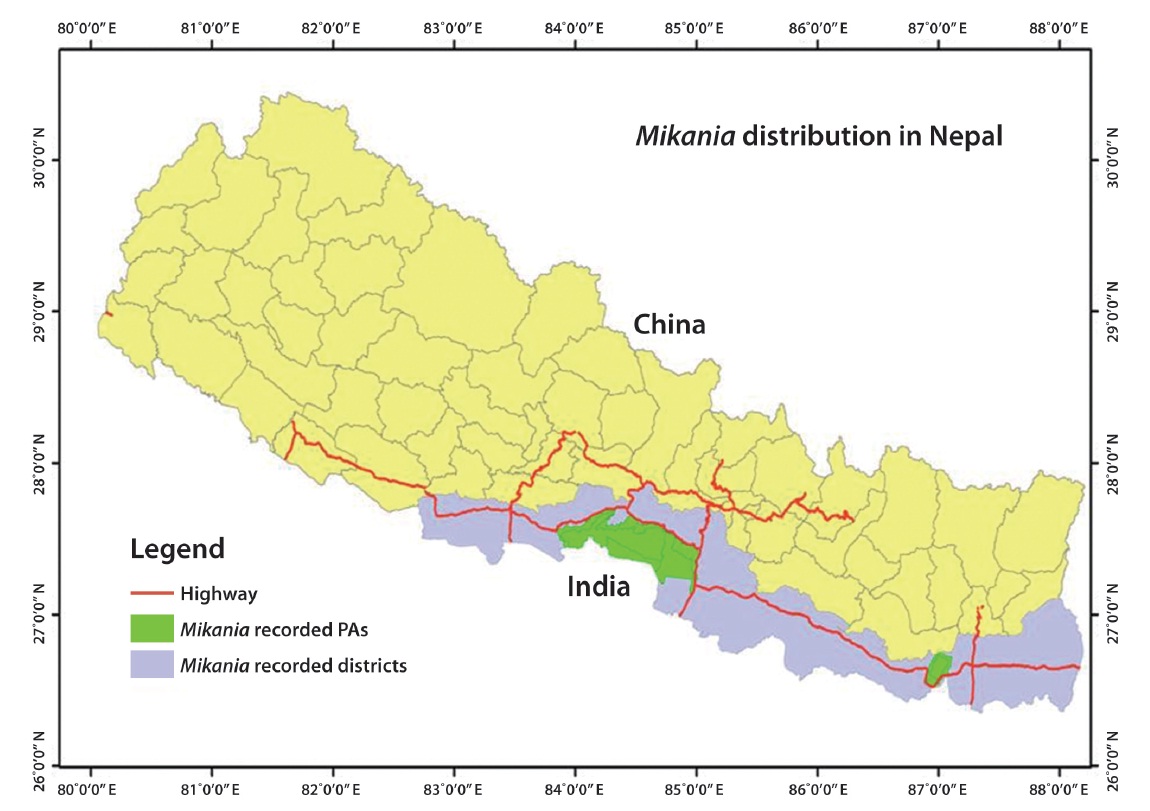
M. micrantha was first recorded in the eastern region (Ilam district) of Nepal in 1963 before spreading westwards (Tiwari et al. 2005). The vine is now recorded in 20 eastern, central, and western Terai districts of Nepal,
including three protected areas (PAs) such as Chitwan National Park, Parsa Wildlife Reserve, and Koshi Tappu Wildlife Reserve (Fig. 1). M. micrantha is considered the most problematic terrestrial invasive plants in the tropical parts of Nepal (Poudel et al. 2005). Mikania has influenced over 100 native plant species, particularly plants with a diameter at breast height < 30 cm in the buffer zone of Chitwan National Park (Sapkota 2007).
The abundance of invasive plants usually reduces the availability of native plants, which can influence the delivery and quality of forest products, and ultimately affect the livelihood strategy of rural farmers, as forest products are the major farm household production input. This is because plant species richness and ecosystem services are intricately linked, and a change in the state of one of these variables can be expected to have an impact on the other (Costanza et al. 2007). In general, the vulnerability of rural livelihoods and control costs increase with increased abundance of invasive plants (Shackleton et al. 2007).
Similar to other developing countries, Nepal has not prioritized control of invasive species as one of its conservation strategies (Nun ez and Pauchard 2010). The absence of a control strategy has permitted M. micrantha to propagate freely. As M. micrantha has a tremendous growth rate and can regenerate from both seed and vegetative parts, rural farmers consider controlling the spread of M. micrantha as an unattainable mission (Rai et al. 2012). Various techniques are in practice to control the spread of M. micrantha in different parts of the world. Most control methods can be classified into mechanical, biological, and chemical control.
Control methods using chemicals do not distinguish between target and non-target species (Zhang et al. 2004). The use of toxic chemicals can also have adverse impacts on native vegetation and can contaminate groundwater and river run-off. Classical bio-control has been widely used to control the spread of M. micrantha (Abraham et al. 2002, Zhang et al. 2004). However, there are potential risks of introducing new agents that could attack native species, influence the food chain, and become invasive (Miao et al. 2012). Thus, a mechanical technique such as consecutive cutting of vines at regular intervals could be appropriate to control the growth of M. micrantha, as it focuses only on the target species (Kuo et al. 2002, Lian et al. 2006).
An effective management strategy for invaded forests should not only focus on eradicating an invasive species but should also enhance the existing native ecosystem. Thus, manual cutting could be an appropriate strategy; however, it demands significant amounts of labor and time. Manual cutting targeting M. micrantha will contribute to controlling its growth and create favorable conditions to promote regeneration of native species.
Manual cutting for the situation in Nepal is appropriate because of: (i) the availability of cheap labor; thus, the government can implement a large-scale control program, (ii) local forest users are managing about one-fourth of the country’s total forests, so they can participate in the M. micrantha cutting operation, and (iii) manual cutting has no side effects. However, the implementation of control activities including uprooting, cutting, and burning by local forest user groups failed to produce the expected results in the buffer zone of Chitwan National Park, Nepal (Rai et al. 2012). In this context, this study seeks to answer two research questions by establishing experimental plots in the Janakauli buffer zone community forest, Chitwan, Nepal in August 2011. First it considers, why did manual cutting employed by local farmers fail to control M. micrantha re-sprouting? Second; how much will the cutting operation cost?
Two sites were selected to establish experimental plots. Selection was based on canopy coverage such as (i) open woodland and (ii) closed woodland. Open woodland was defined as a forest site with canopy coverage < 20%, otherwise it was a closed woodland. Two types of cutting strategies (CS) were used: CS (i) was cutting M. micrantha only, and CS (ii) was cutting all vegetation in the regeneration layers except tree and shrub seedlings. Four different treatments were applied to assess the performance of cutting based on the cutting intensity as practiced by Kuo et al. (2002). These treatments were: control plots with no cutting intervention (T0), cutting once (T1), cutting twice (T2), and cutting three times (T3) during the experimental period. The time interval between two consecutive cuttings was 3 weeks.
The treatments were assigned following a complete block design. Thirty-two experimental plots were laid out in the open woodland, and the size of the experimental plots was 5 m × 5 m. Both types of cuttings were applied. Hence, in open woodland, the block with cutting strategy (i) had 16 experimental plots and the block with cutting strategy (ii) had 16 experimental plots. Every treatment cutting strategy group had four replications. The size of the experimental plots in the closed woodland was 10 m × 10 m, and only cutting strategy (i) was carried out. It had eight plots and all treatments were applied twice.
After laying out the experimental plots, a baseline vegetation condition assessment was carried out. First, M. micrantha vines and seedlings were counted, and coverage of M. micrantha and canopy openness in each plot was estimated. M. micrantha coverage was divided into four categories based on the coverage of vines: category I, up to 30%; category II, 31-60%; category III, 61-90%; and category IV, > 90%. Canopy openness was estimated from each of the four corners of each plot by the naked eye and added. Then, the open canopy average was estimated by dividing by four.
First, cutting was carried out in the plots assigned to the T1, T2, and T3 treatments. Only the plots assigned to the T2 and T3 treatments were treated during the second cutting, and only the plots assigned to the T3 treatment were treated in the third cutting. During each cutting operation, the number of M. micrantha and seedlings of native species were counted, and harvested fresh biomass was weighed. The fourth cutting was carried out in all experimental plots, the removed fresh biomass was weighed, and native species seedlings were counted.
All tests were carried out at a P < 0.05 significance level using SPSS ver. 18 (SPSS Inc., Chicago, IL, USA). Results from the completely randomized design were analyzed with a one-way analysis of variance (ANOVA) (least significant difference [LSD]). In particular, the effects of different cutting intensities on regeneration of M. micrantha and other native species was the main interest, and analyses were carried out individually for each block. A multiple regression was performed to understand the factors affecting a change in regeneration of M. micrantha. The following regression model was used:
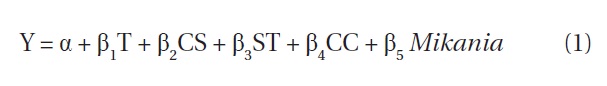
where, Y is the change in M. micrantha (expressed in %) after cutting operations, α is a constant term, T is treatment type (values of 1, 2, 3, or 4 for T0, T1, T2, and T3) respectively, CS denotes the cutting strategy which was 1 for cutting strategy (i) and 0 for cutting strategy (ii), ST denotes the site type which was 0 for open canopy and 1 for closed canopy, CC denotes crown coverage of the experimental plots expressed as a percentage, Mikania is the number of M. micrantha plants per plot recorded during the baseline vegetation assessment, and β1 to β5 are the vectors of the coefficients.
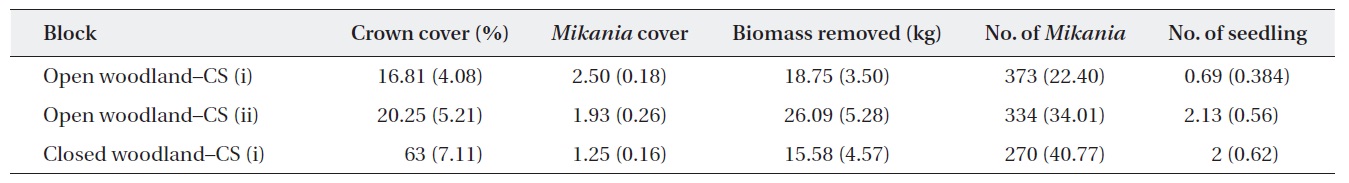
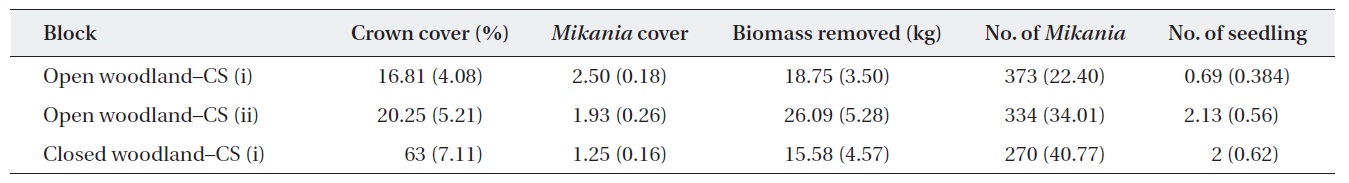
Baseline information on the experimental plots showed that the average M. micrantha coverage and the number of plants in the open woodland were higher than those in the closed woodland (Table 1). The baseline statistics indicated that M. micrantha coverage had an inverse relationship with the crown cover of the experimental plots. The average fresh biomass removed during the first cutting was reported, and, as expected, the highest amount of biomass removed from the open woodland plots was achieved with cutting strategy (ii).
We assumed homogeneous site quality, as soil characteristics of a site are less important for growth of M. micrantha (Wen et al. 2000). Light condition plays an important role in M. micrantha growth, as the vines are strongly light demanding (Ipor 1991). They generally colonize aquatic ecosystems such as marshes and riverbanks, and rarely grow in other habitats (Ye and Zhou 2001). During the field work, we observed the abundance of M. micran-
tha in riverine forests and forest patches close to water sources.
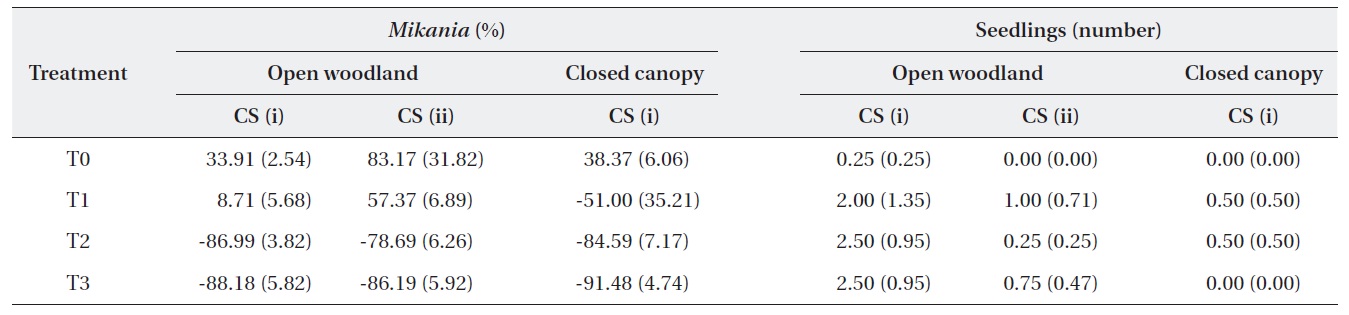
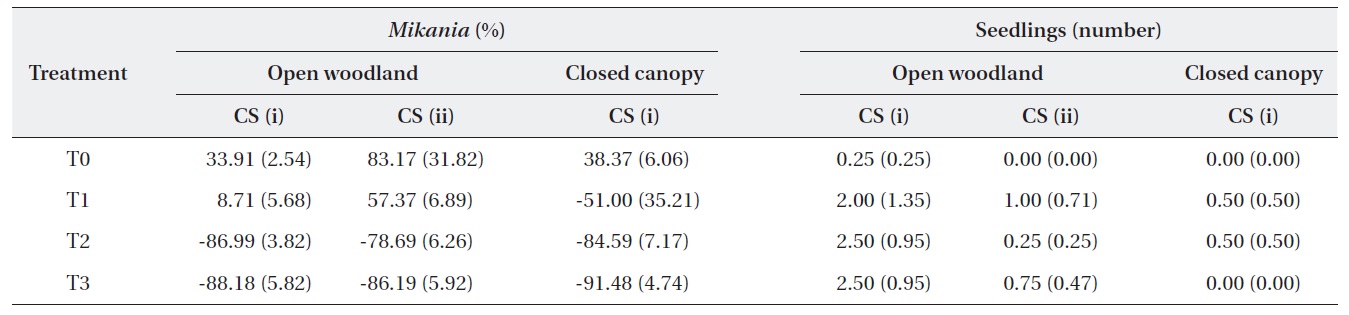
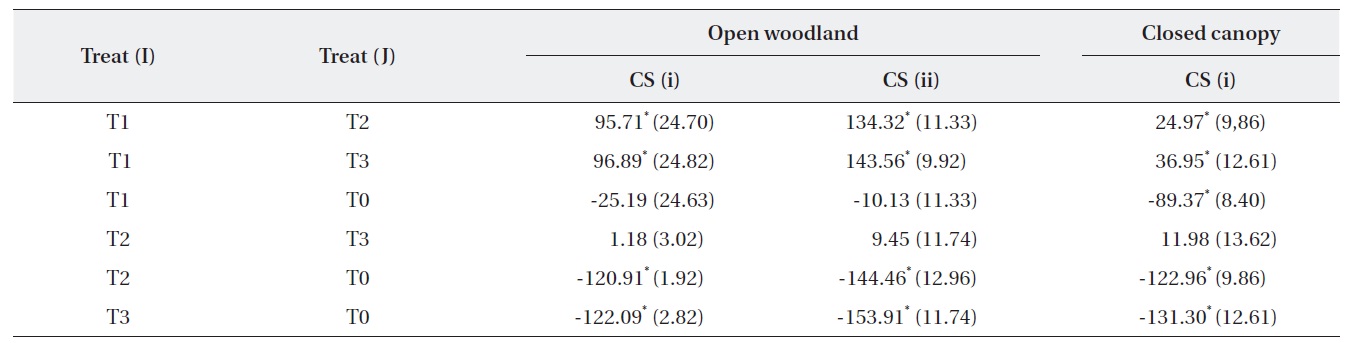
The change in the number of M. micrantha by percentage in different blocks was estimated (Table 2). The results show that three consecutive cuttings eliminated up to 91% of the vines. Additionally, the trend showed that vine mortality increased with the number of consecutive cuttings. The statistics of control plots (T0) indicated that the abundance of M. micrantha increased over time in the absence of a control program and also showed the plant’s aggressive proliferation. Interestingly, a single cutting operation enhanced regeneration of M. micrantha in the open woodland block under cutting strategy (ii). This cutting operation was similar to the local practices carried out as a control strategy.
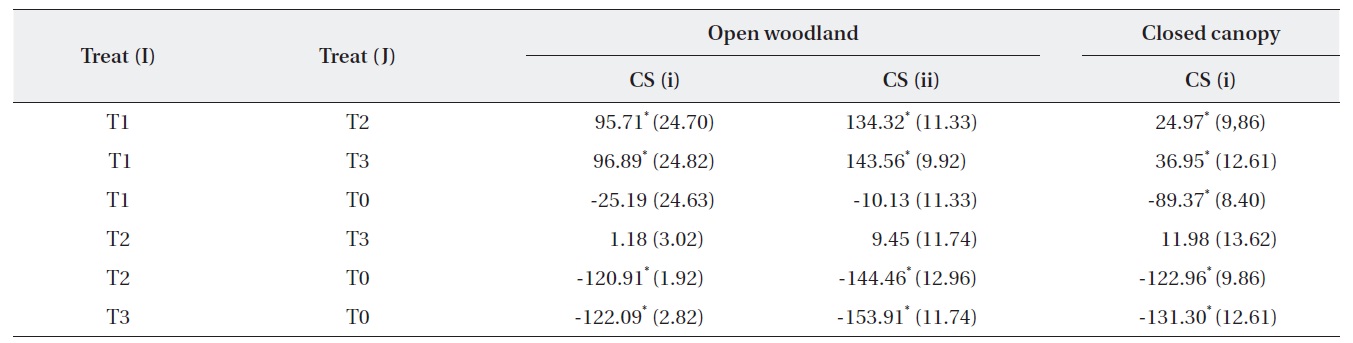
A one-way ANOVA (LSD) was performed to examine the significance between the changes in the number of M. micrantha plants resulting from the different cutting treatments (Table 3). The change in the number of M. micrantha under different cutting treatment plots, particularly T2 and T3, was statistically significant compared with that in the no-cut plots (P < 0.05). The effects of cutting twice and three times were significantly different from the effects of cutting once in all three blocks. But, no significant difference was observed between the effects of cutting twice or three times. The change in Mikania in the T1 experimental plots was not significant compared with the changes that occurred in the no-cut plots (T0) in the open-woodland, regardless of cutting strategy.
A change occurred in the number of native species seedlings in the different treatment plots (Table 2). The results showed positive effects of the cutting treatments on the regeneration of native plants. However, the changes were not statistically different, based on a one-way ANOVA (LSD). This may have been due to the time span of the study. In the long run, it would be expected that cutting M. micrantha provides space for regeneration of native plant species. However, this speculation requires further research conducted for a longer duration.
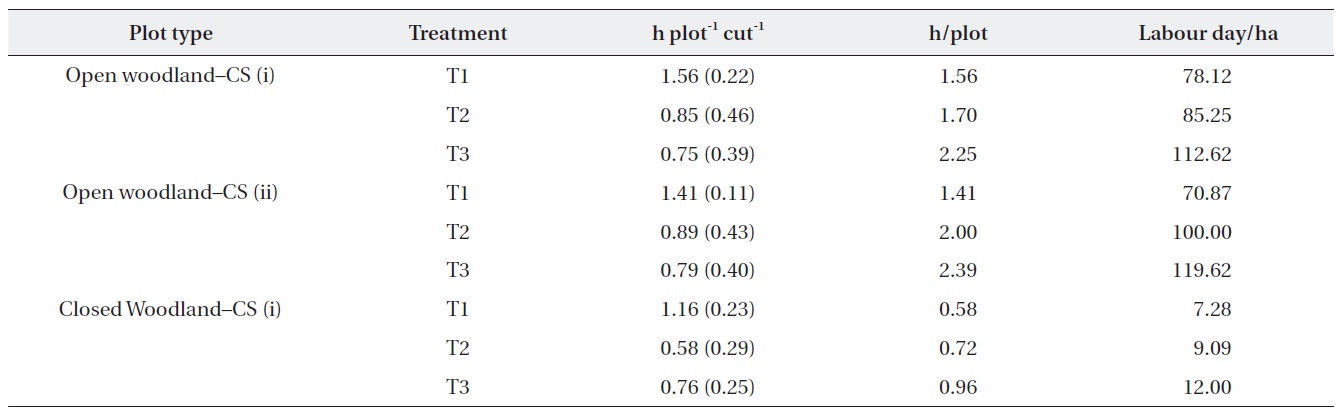
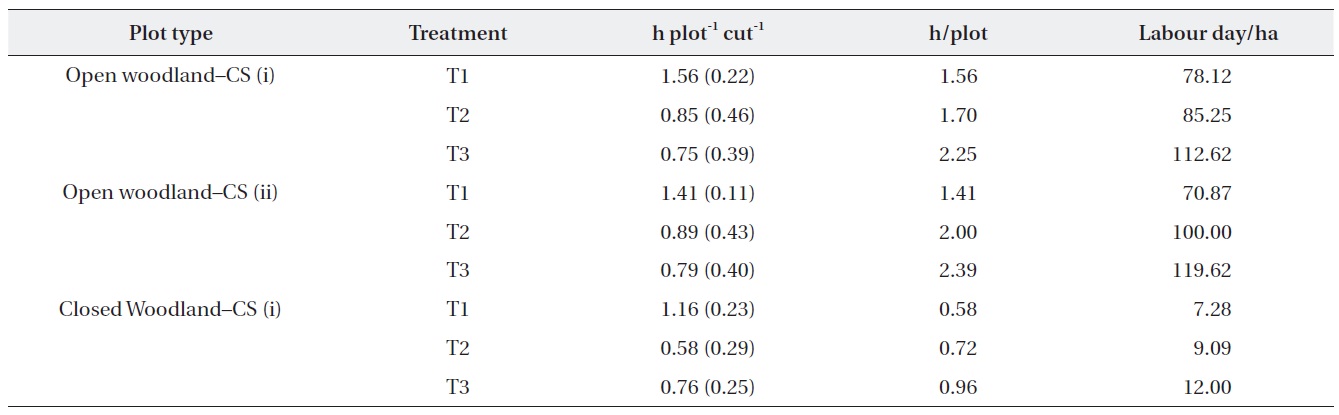
The costs of the operation were estimated based on the actual time taken to cut (Table 4). The results showed that the time required for the operation depended on the
abundance of M. micrantha. The costs of operation in terms of labor hours in the open woodland were substantially higher than that in the closed woodland. In addition, the number of labor hours required per plot in the different treatment plots showed that the first operation was the most expensive. Strategy (i) of removing M. micrantha only was more expensive than cutting strategy (ii), which removed all ground vegetation except tree and shrub seedlings. However, cutting strategy (i) was cheaper for subsequent cutting operations than that of strategy (ii). We assumed that one labor day was 8 h for this estimate.
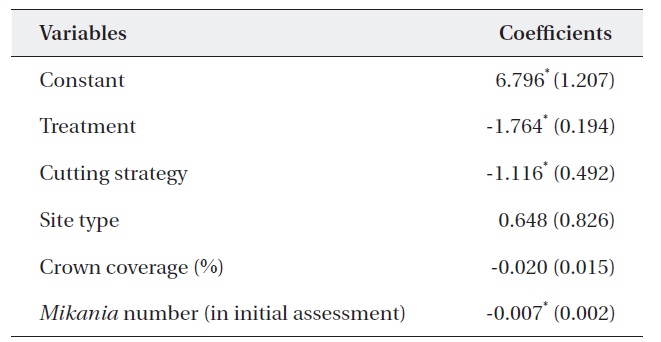
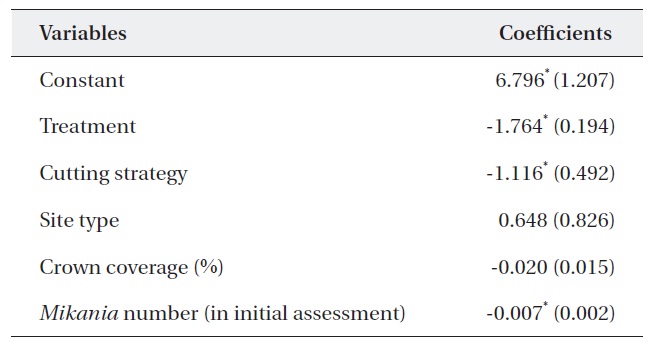
Table 5 shows the results of a multiple regression analysis. The dependent variable was the percent change in M. micrantha. The r2 value of 0.737 indicated that the model was a good fit. This result shows that variables including
treatment, cutting strategy, and the number of M. micrantha vines in the initial assessment were major determinants of changes in the number of vines.
The negative sign for treatment indicates that mortality of M. micrantha increased with the number of consecutive cuttings. This finding supported previous results (Kuo et al. 2002, Lian et al. 2006). Similarly, the negative sign of the cutting strategy indicated that removing only M. micrantha only reduced the number of M. micrantha plants significantly compared to removing all vegetation except trees and shrubs. This could be due to the light intensity on the ground, as the M. micrantha only plots had a ground cover of shrubs, which may not pass enough light, resulting in higher rates of mortality compared to cutting strategy (ii). At the same time, removing all vegetation can offer suitable habitats for M. micrantha due to increased light intensity and encourage growth and survival.
Similarly, cutting was more effective in the plots with an abundant number M. micrantha. The insignificant coefficient of the site type indicated that cutting intensity had the same effect in both open and closed canopy plots. Therefore, forest managers do not require a specific cutting strategy based on canopy coverage. However, it should be noted that a low abundance of M. micrantha in the closed canopy may produce less effective results than that in open woodlands.
The results showed that manual cutting reduced the competitiveness of M. micrantha substantially and effectively control proliferation. As expected, the open woodland had higher M. micrantha abundance than that of the closed woodland because of the light loving nature of the vines. However, the effects of manual cutting were identical across the experimental plots regardless of canopy openness. The results also demonstrated that removing the vines created favorable conditions for native plant species and promoted their recovery (Lian et al. 2006). In general, the abundance of M. micrantha reduced the availability of native species and vice versa.
This study also answered why control strategies practiced by local forest users failed to produce expected results (Rai et al. 2012). There are two main reasons behind the failure of local manual cutting practices. First, there was a seasonal effect, as forest user groups were practicing control measures during December and January. However, although winter is the ideal season for forestry operations including thinning and pruning, this method is less effective to control the spread of M. micrantha (Kuo et al. 2002). Because winter is the peak flowering season of the vines, insitituting a cutting operation during this season may help to spread seeds instead of eliminating M. micrantha. Second, no periodic cuttings were conducted, as forest users were cutting the vines only once per year. Our results showed that a single cutting operation may promote vine proliferation. In general, invasive plants perform better in disturbed habitats and are highly competitive to recover following cutting (Lian et al. 2006). However, repetitive cutting can hinder their growth (Feng et al. 2002, Kuo et al. 2002).
M. micrantha vines usually colonize an open area with moist soil (Willis et al. 2008), indicating that manipulating light conditions can help to control vine proliferation. The results showed that removing M. micrantha only from the ground cover produced better results than removing all herbs and climbers from the ground in terms of eliminating vines. The cutting strategy retaining native ground cover limited the availability of space and light for germination. Cutting strategy (i) could be appropriate to manage invaded grasslands, where ground cover plays an important role manipulating site conditions to degenerate M. micrantha. However, this cutting strategy is more costly during the first cut, but the subsequent cutting operations become comparatively cheaper with the reduction in the number of vines. As the costs of control measures depend on the abundance of the invasive species, in the long run, the cutting strategy of removing M. micrantha only becomes more economically efficient to control the vines compared to the cutting strategy of removing all shrubs and climbers from the ground (Shackleton et al. 2007).
The estimates of control costs indicated that the cutting operations were not as expensive as expected in the forest patches with crown cover > 60%. According to local forest users, the costs of the uprooting operation was NRs (Nepalese Currency-Nepalese Rupees (NRs); 1 US$ -NRs. 81.00 during the experimental period). 10,000 per ha, which is equivalent to 28.57 days per ha at local wage rate (NRs, 350/day). However, the open woodland demanded a substantial amount of labor . On average, the community forest area per household is 0.73 ha (Do 2008). This means for the first year, each household should spend at least 8.76 to control the growth of M. micrantha in the invaded area, for a three consecuting year cutting operation to be carried out. The labor requirement could be less when it is implemented in a larger area, as this is based on the estimates from the experimental plots.
The results of our study suggest that controlling the infestation of M. micrantha through manual cutting is a gradual process. Regular removal of M. micrantha decreases the abundance of the vines and promotes regeneration of native plant species. In the long run, it modifies understorey shade, which is desirable to reduce the competitiveness of M. micrantha, and ultimately contribute to control further regeneration (Feng et al. 2002).
We concluded that manual cutting could be an appropriate strategy to maintain the native ecosystem in an invaded area by constraining the growth of M. micrantha. Regular cutting operations enhance understory shade and promote the regeneration of native plants, which is the desirable condition to impede the growth of lightdemanding species. In general there is a risk to introduce new biological agents to control the invasive species; therefore, manual cutting seems to be an appropriate strategy to control M. micrantha growth. However, the following three points should be considered:
- Cutting above ground biomass should start before flowering, and M. micrantha flowering starts in September in Nepal.
- There should be at least two consecutive cuttings in a 3-week interval.
- Only M. micrantha should be removed during cutting, and native ground cover should be retained.
Admittedly, the mortality of M. micrantha increased with increased frequency of cuttings; however, selecting two or three cuttings depends on the available resources. In general, cutting costs can be minimized by utilizing the removed plant materials. Empirical studies have shown that invasive plants can be transformed into economic goods though appropriate human intervention (Kaufmann 2004, Rai et al. 2012). Further study is needed to explore possible uses for the harvested M. micrantha biomass.
The spread of M. micrantha has a clear trend of moving west in Nepal. Bare ground should be minimized to control its further spread (Feng et al. 2002). There is a need to assist local forest user groups to include invasive species management activities in their operational plans. These above mentioned points should be followed while preparing such activities.