The exploitation of high-power secondary batteries has received considerable interest as part of efforts to solve environmental and energy problems while promoting social develop-ment. Lithium ion secondary batteries are one of the most important rechargeable batteries, and are widely used nowadays as power sources for portable electronic devices such as laptop computers, cellular phones and camcorders, due to their high energy density. Large size lithium ion batteries are also very attractive for use in hybrid electric vehicle or for fully electrical vehicle applications. The application requires high power, high energy density and a high efficiency power supply [1-3]. Studies on anode mate-rials, aimed at improving material capacity and cyclability, have been active for many years [4-9].
The electrochemical properties of natural graphite anodes, involving initial efficiency, re-versible capacity and cycle stability have been improved due to higher graphitization degree, higher purity, more suitable particle size distribution, and so on. Recently, natural graphite has come to be considered as a promising anode material for lithium ion batteries due to its high reversible capacity, appropriate charge/discharge profile and low cast. However, low first cycle efficiency and poor cycle stability have limited its practical use [10,11].
Wang et al. [12] demonstrated that mechanical milling of natural graphite could be one possible way to produce disordered carbon atoms with large intercalation capacity. However, mechanically milled samples often have very large specific surface areas, leading to extensive irreversible reactions during the first charge.
The treatment of graphite with oxidative solutions of H2O2, Ce(SO4)2, HNO3 and (NH4)2S2O8 resulted in marked improvement of its electrochemical performance as anode material for lithium ion batteries [13]. According to Wu et al. [13], the improvements might be due to the elimination of structural imperfections that have high levels of activity toward lithium, such as carbon chains, the introduction of more micropores/nano-channels, the modification of the surface of the graphite with a cover of a dense layer of oxides, and the improvement of the stability of the graphite structure.
Yang et al. [14] reported that the electron conductivity of multi-walled carbon nanotubes (MWCNTs) was around (1~4) ×102 S/cm along the nanotube axis and 5-25 S/cm perpendicular to the axis. However, the conductivity of carbon black is around 2.1 S/cm. The electronic connections would be improved when MWCNTs are used instead of carbon black in the anode preparation. Li et al. [15] performed a comparative investigation of carbon black and MWCNTs as conducting additives, and had results that showed that the addition of MWCNTs remarkably increased the reversible capacity and cycling stability.
In this study, the objective is to improve the performance of graphite anodes by modifications such as optimum milling con-ditions, proper acid treatment, and introducing various carbon additives. In addition to a single treatment of acids or carbon ad-ditives for the graphite anodes, a mixture of different carbon ad-ditives was also applied. By changing the mixing ratio of carbon black and CNT, the obtained composite anode materials were tested to investigate the electrochemical characteristics.
2.1. Preparation and characterization of anode materials
The flake graphite (FG, 10~15 ㎛ of thickness, hundreds of㎛ of width) particles for the anode materials were prepared by pulverizing natural graphite with a planetary milled for 2~9 h and sieving it at 325 mesh. The spherical graphite (SG, 20 ㎛ of diameter) particles were treated with 10 wt% of HNO3 and H2SO4 for 2 h. Acetylene black (AB, S. A. 60~80 m2/g; Alfa Ae-sar, USA), conductive carbon black (Super P, TIMCAL, Swit-zerland), Ketjen black (KB) ethylene carbonate (EC, 600JD, S.A. 1270 m2/g, P. H. Chemical) and CNT (diameter 10~15 nm, Hanwha Nanotech, Korea) were introduced to SG particles by 1 h mixing with a magnetic stirrer. The contents of the additives in the SG anodes were varied in the range of 3~7 wt%. The mixing ratios of the CNT to the conductive carbon black were varied, with the following values: 1:4, 2:3 and 1:9 at 5 wt% of total ad-ditive content.
The electrodes were prepared by mixing 93 wt% of the active anode materials and 7 wt% of polyvinylidene fluoride binder dissolved in 1-methyl-2-pyrrolidinone. The mixed slurry was then coated on copper foil by a Doctor Blade Method and dried at 80oC in a vacuum oven for 24 h before being compressed by a roll press at 80oC. The prepared anodes in a 2.5 × 2.5 cm size were kept in a glove box filled with argon. The prepared elec-trode was used as a working electrode and lithium foil adhered to a copper mesh with dimensions of 3.0 × 3.0 cm was used as a counter electrode. 1 M LiPE6 salt dissolved in a mixture of EC, ethyl methyl carbonate, and dimethyl carbonate at 1:1:1 volume ratio was used as the electrolyte. The half cells were assembled in a glove box with an argon atmosphere using a polypropylene (Celgard® 2400 microporous membrane) separator to separate the cathode and the anode.
2.3. Analysis of electrochemical properties
The electrochemical properties of the half cells were esti-mated by using a WBCS-3000 Battery Charge/Discharge Cycler (Won A Tech Co., Korea) at a constant temperature of 25°C. The charge characteristics were measured by applying the constant current (CC) method at 0.2 C for the first charge until reaching 0.005 V and then changing to the constant voltage method for the successive charge by reducing the current until 2.5% of the initial current was attained. For the discharge, the CC method was applied at 0.2 C until reaching 2 V. The current rates in the CC method increased to 1, 5 and 10 C for the high rate charge-discharge characteristics. 1 C means a rate of current at which the full charge or discharge for the given anode sample would take 1 h, assuming 300 mAh/g of capacity.
The microstructures of FG and SG were observed by a scanning electron microscope (SEM, 3500N, Hitachi Science System Ltd., Japan). The BET surface areas were measured by using an auto-matic surface area analyzer (Micromeritics, ASAP 2020).
3.1. Natural FG as active anode materials for Li insertion
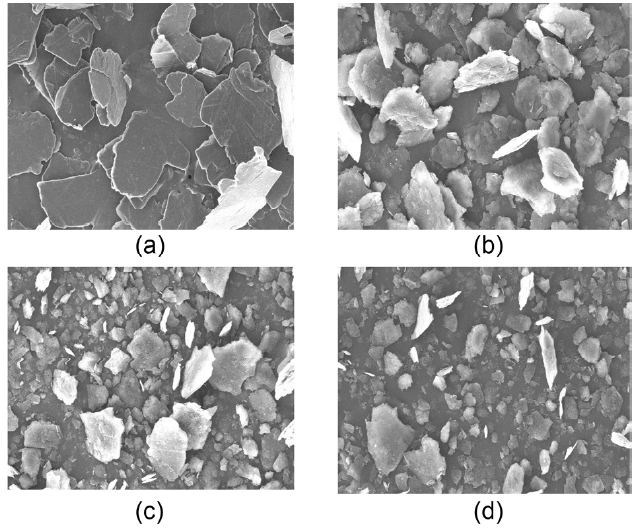
Fig. 1 shows the SEM images of natural FG before and af-ter milling with the planetary mill. It can be seen that the par-
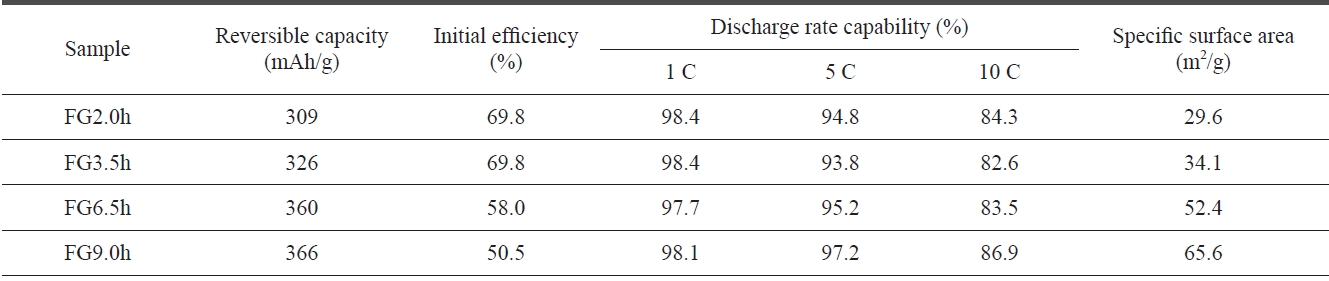
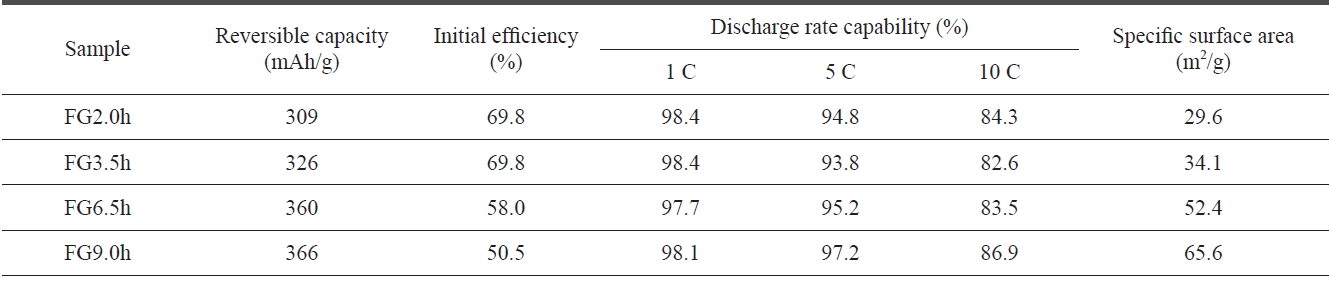
[Table 1.] Discharge characteristics of flake graphite using different milling times
Discharge characteristics of flake graphite using different milling times
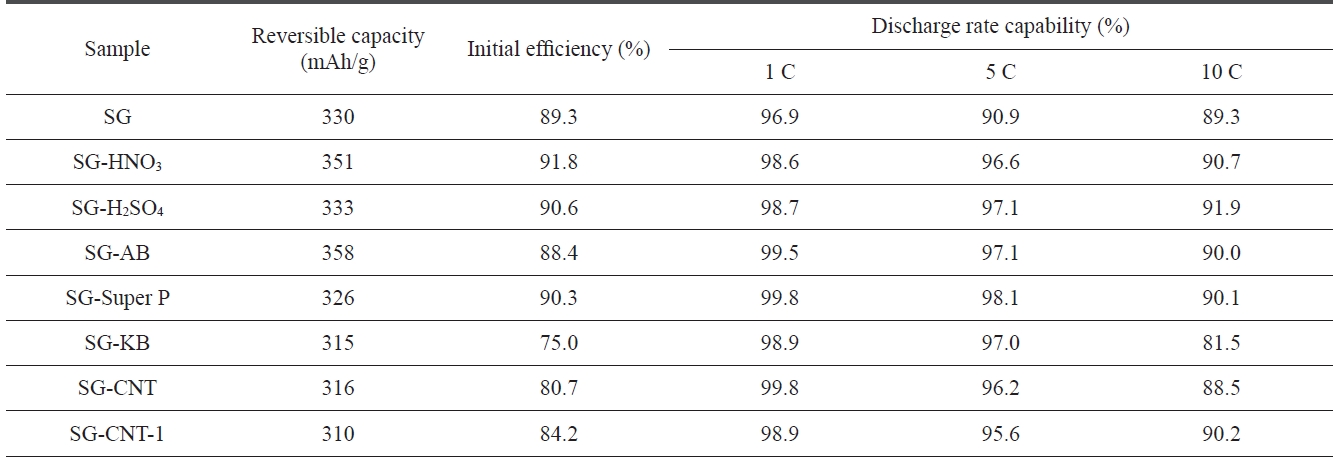
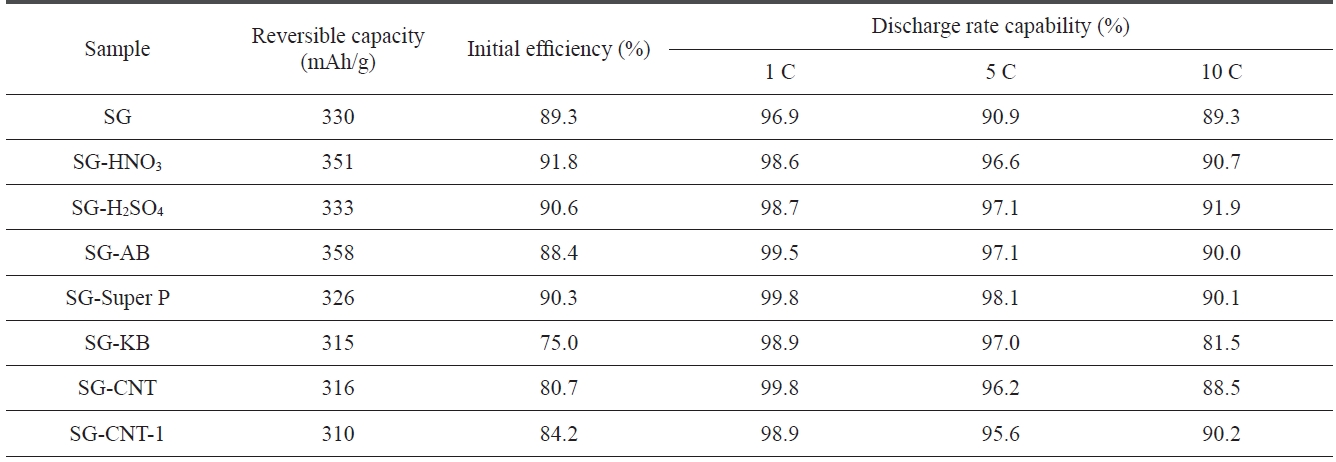
[Table 2.] Performance of SG and SG treated with different acids or additives
Performance of SG and SG treated with different acids or additives
ticle sizes of the FG became smaller with increased milling time. The milling treatment was able to cut the graphite par-ticles into smaller pieces in directions both perpendicular and parallel to the basal planes [6]. Table 1 shows the discharge characteristics of the FG as a function of the milling time. It can be seen that the discharge capacity increased but that the initial efficiency decreased with the longer milling time. It can be conceived that a smaller particle size is favorable for high reversible capacity because of the larger exposure of the edge planes toward the electrolyte. As expected, the specific surface areas increased with the longer milling time, as shown in Table 1. However, the fine-particles of natural graphite will inevitably incur very large irreversible capacity due to the increased surface area, which will then decrease the coulombic efficiency [12].
3.2. SG with a single treatment of acids or car-bon additives as active anode materials
Table 2 shows the discharge characteristics of SG anodes us-ing different acids and carbon additive treatments. The discharge capacity increased with the HNO3 treatment, and the discharge rate capability and the initial efficiency improved slightly with both acid treatments. These enhancements may be due to the elimination of structural imperfections, which results in high ac-tivity toward lithium ions.
It can be seen that the initial efficiency of SG did not change significantly with the AB or Super P additives but that it did decrease slightly with the CNT additive. The discharge rate capability of AB and Super P treated SG improved slightly as compared to that of SG. SG-CNT-1 indicates that CNT was treated with milling for 15 min before it was added to SG. The milling treatment of the CNT improved the discharge rate capability and the initial efficiency of the CNT added SG anodes. This might indicate that the CNTs are significantly shortened and that they changed out of the entangled struc-ture during the milling treatment. The shorted and disentan-gled CNTs help to increase the electrical conductivity within the composites of anode materials, causing them to exhibit better electrochemical performance.
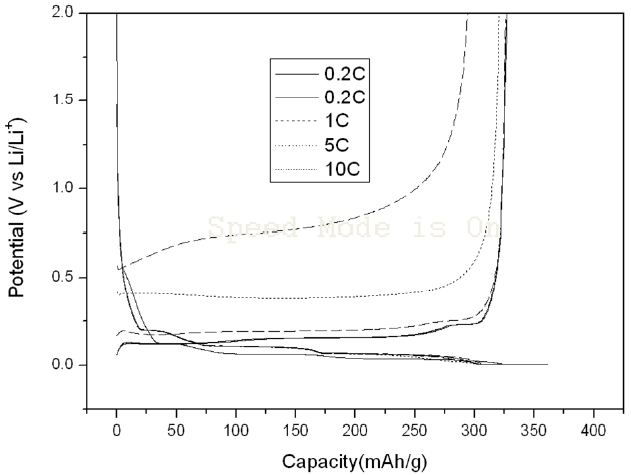
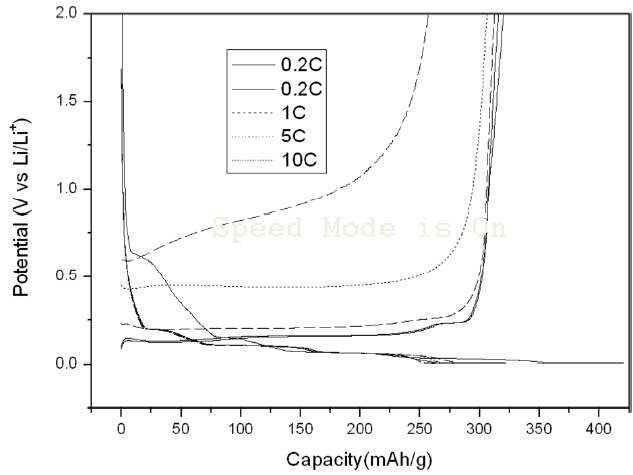
Figs. 2 and 3 show the charge-discharge characteristics of the SG anodes treated with Super P and KB. The left curves present the charge property and the right curves present the discharge property. The discharge curves for Super P treated SG rise more in a more upright way in the range of 0.2~2 V and the discharge capacity was reduced more gradually with the increase of the C-rate up to 10 C, as compared to the case of the KB treated SG. As a result, the discharge capacity of the Super P treated SG at 10 C-rate was much higher than that of the KB treated SG, although their discharge capacities at 0.2 C were in the same range. The charge curve of KB treated SG was more extended than that of the Super P treated SG. This illustrates that the initial
efficiency of the KB treated SG is lower than that of the Super P treated SG.
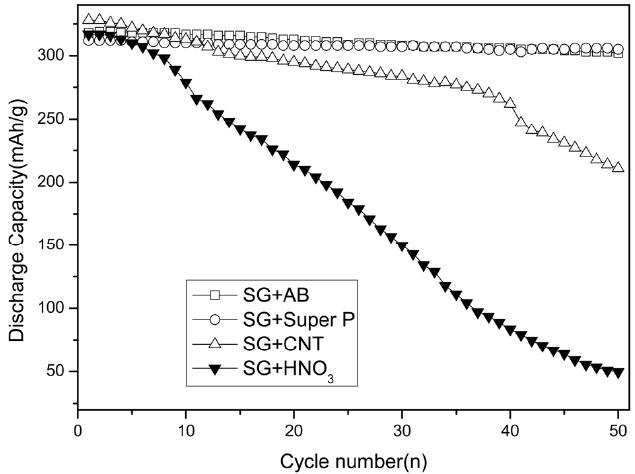
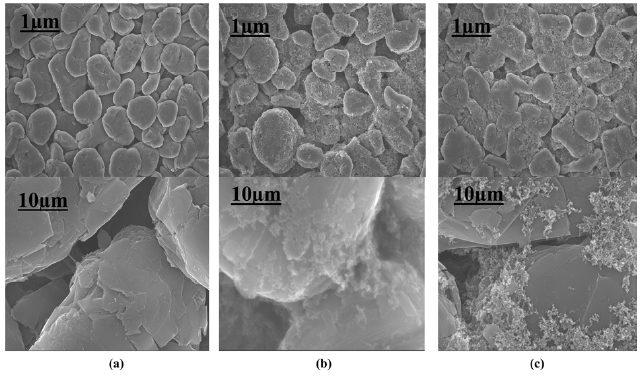
Fig. 4 presents the cyclic performance of the SG electrodes with different additives. The discharge capacity of SG an-odes with the AB or Super P additives was retained even after 50 cycles but that of the SG anodes without the carbon addi-tives dropped dramatically. The conductive additives might be concentrated near the contact points between the two adjacent graphite particles. Fig. 5 shows SEM images of the SG and the SG treated with different additives. It was confirmed that the ad-ditives were connected between the graphite particles, as shown in Figs.5 b and c. These conductive bridges are very effective at improving the electrical contact between the graphite particles during the later cycles.
3.3. SG anodes with different contents or a mixture of additives as active anode materials
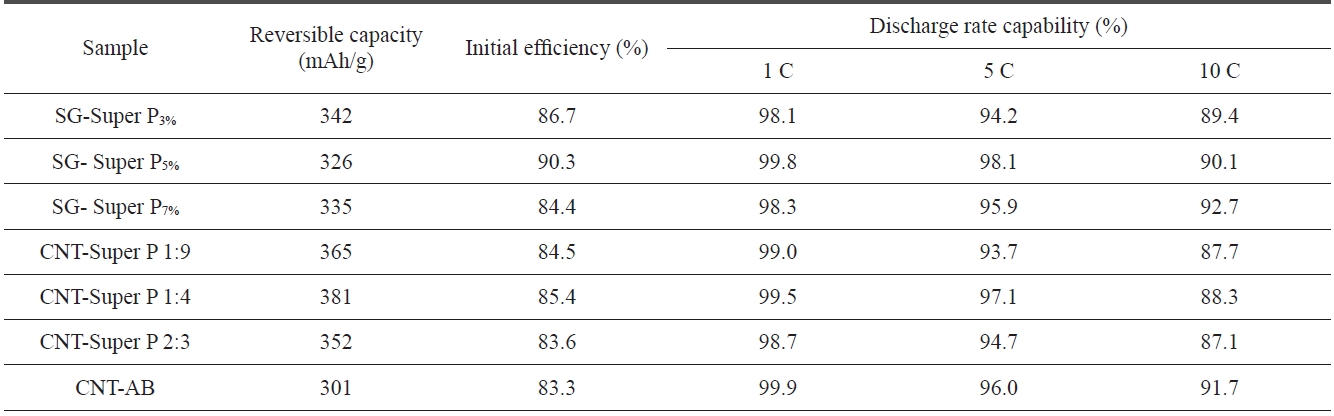
Table 3 shows the performance of SG anodes treated with different contents of Super P and with a mixture of CNT and Su-
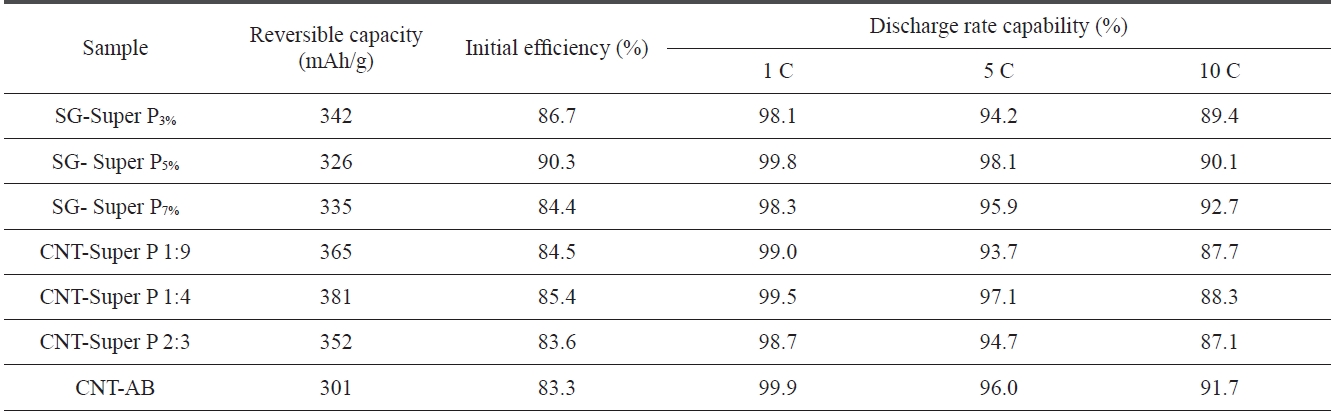
per P. The reversible capacity of the SG anodes did not change significantly with the variation of Super P content. However, the discharge rate capability improved with an increase of the Super P content and the initial efficiency showed the highest perfor-mance at 5 wt of Super P content.
The SG anodes treated with a mixture of CNT and Super P had a relatively high reversible capacity as compared to those treated with a single additive. The reversible capability showed the highest value of 381 mAh/g at a 1:4 ratio of CNT to Super P. However, the SG anodes treated with a mixture of CNT and Super P showed a lower initial efficiency and discharge rate capability than those treated with a single additive. The SG anodes treated with a CNT and AB mixture showed a relatively high 10 C-rate capability of 91.7%.
In order to improve the performance of anode materials for lithium ion secondary batteries, milling treatment for FG, and either acid or carbon additive treatments for SG, were carried out. With the increased milling time, the discharge capacity of the FG increased but the initial efficiency decreased. The
[Table 3.] Performance of SG treated with different contents or mixture of additives
Performance of SG treated with different contents or mixture of additives
HNO3 and H2SO4 treated SG showed a slightly higher revers-ible capacity, a higher discharge rate capability, and higher initial efficiency than the SG without any treatment due to the elimination of structural defects of graphite by the acid treatment. The Super P treated SG also showed a slightly im-proved initial efficiency and discharge rate capability as com-pared to that of the SG without any treatment. The SG anodes treated with a mixture of CNT and Super P had a relatively high reversible capacity as compared to that of anodes treated with a single additive. The SG anodes treated with a mixture of CNT and AB showed a higher 10 C-rate discharge capabil-ity than those treated with a single additive. With treatment with carbon black additives such as AB or Super P, the dis-charge capacity was retained even after 50 cycles. The con-ductive additives and binder might become concentrated near the contact points between the two adjacent graphite spheres and these conductive bridges will be very effective for im-proving cyclability.