Atmospheric air is a unique source of oxygen required by humans for respiration. It should be free from toxicants and in breathable form to support life. Contamination of the same can occur either intentionally or accidentally, which can make life difficult. Chemical and biological contaminants with toxic properties have been abused as debilitating agents and have been identified as chemical and biological warfare (CBW) agents [1]. These chemical weapons, when deployed, rapidly fall on to the ground and exist in the environment as aerosols, vapours, and gases, causing incapacitation, and aiding in disarming and capturing the enemy [2].
The only way to overcome the adverse affects of CBW agents is to take proper protective measures. Respiratory protection is of great importance as breathable air is the primary requisite for any individual; such protection can be provided by the gas mask and canister. The performance of the canister mainly depends on the particulate and gas filters. A commonly used particulate filter is the high efficiency particulate aerosol (HEPA) filter media.
Active carbon of high surface area has been widely used for the purification of air and water owing to the adsorption capacity of this universal adsorbent [3-5]. Active carbon removes a wide range of chemicals on the basis of physisorption, which depends on its porous structure. In order to increase the capacity of active carbon for adsorbate degradation it is pre-impregnated with chemicals [6] that add chemical degradation properties to the physi-sorption capacity of carbon.
The impregnated carbon is used in nuclear, biological, chemical (NBC) canisters and filters for the removal of persistent and non-persistent chemical warfare (CW) agents. Persistent CW agents such as blister and nerve agents are mainly held over the adsorbent surface through physical adsorption, while nonpersistant gases such as hydrogen cyanide, cyanogen chloride, and phosgene are converted into non-toxic products by their chemical reaction with impregnated metal salts [4,7-9]. Ideally, the filtration systems should be of such a type that they can provide filtered air against the whole spectrum of chemical warfare agents and not pose any problem of cross contamination; they should also ensure safe handling and disposal. Therefore, there exists a need to develop an NBC canister capable of chemically degrading all types of CW agents into non-toxic products. It is also necessary to evaluate the developed NBC canister against CW agents.
Indigenously developed NBC canisters have been evaluated against carbon tetra chloride (CCl4) or chloropicrin vapours [3] in order to asses these canisters for their protection capacity against CW agents such as blister agents and nerve agents of high boiling point. CCl4 vapour has long been used as a simulant for CW agents for testing breakthrough times of gas mask cartridges and canisters in the National Approval Test of Respirators [2,10,11]. CCl4 vapour breakthrough behaviour of an NBC canister is also of great interest when considering respiratory protection.
The requirement of the National Approval Test System was to maintain the continuity of the quality criteria of gas masks through the use of a representative organic vapour for testing breakthrough times of gas mask cartridges and canisters. Every compound has its distinctive adsorption or reactive behaviour with a given adsorbent; therefore, the most useful tests of removal efficiency involve the representative organic vapour to be trapped. Similarity of vapour pressure, water-insolubility, and technical feasibility in generating a test airflow containing the vapour ranging from low to high concentration, and a low risk of toxicity are the primary requirements in attempting to single out a CW replacement vapour.
CCl4 vapour has also been used for laboratory measurements of adsorbent efficiencies and retentions [2]. Testing of NBC canisters (to be used against CW agents) with a simulant such as CCl4 vapours has several advantages; for example, the test agent has a low toxicity, requires fewer handling precautions, needs fewer special facilities, and has no need for licensing for field use. Such a vapour also allows more frequent tests due to the lower cost of the test agent. There is no CW contamination of the test bed and no release of CW vapour into the effluent [12]. Besides these advantages, the deterioration of the gas removing capacities of cartridges and canisters due to airborne moisture can also be detected by using CCl4 vapours in the test method.
Active carbon coconut shell origin, 90 CTC of 12 × 30 BSS (1.40-0.50 mm) particle size and surface area about 1259 m2/g, was procured from M/s Active Carbon India, Pvt. Ltd. (Hyderabad, India) and was impregnated with CuCO3.Cu(OH)2, CrO3 and AgNO3, NaOH, and C5H5N. CCl4 (AR grade) was obtained from M/s SD Fine Chemicals. A Mini RAE 3000 VOC monitor from M/s RAE Systems (USA) was used for the detection of CCl4 vapour and a KDS dual- syringe pump series 200 was purchased from Sigma-Aldrich (India).
2.2. Characterization of impregnated carbon
Impregnated carbon contained in the NBC canister was characterized prior to its being injected into the NBC canister. Surface area, micro pore volume, cumulative pore volume, and pore size distribution were measured by nitrogen adsorption at 77 K using an Autosorb 1C from Quantachrome Instruments (USA). The surface area was calculated using the Brunauer-Emmett- Teller (BET) method. Micropore volume was calculated with the Dubinin- Radushkevich equation (DR) method and pore maxima for micropores were calculated using the Mikhail, Brunauer, and Bodor (MP) method. The cumulative desorption pore volume was obtained with the Barrett, Joyner, and Halenda (BJH) equation.
Metal ion loading was determined by extracting metal ions from the impregnated carbon in acidic and alkaline media. Copper (Cu), Chromium (Cr), and silver (Ag) ions were extracted as CuCl2, Na2CrO4, and Ag2S2O3 using concentrated HCl, NaOH, and Na2S2O3, respectively, and thereafter estimation was performed with an AAS, GBC Avanta PM, Australia, as well as by the titrimetric method. The extracted concentrations of metal ions were found to be very close to the calculated loading in the impregnated carbon. For scanning electron microscopic (SEM) characterization, the powder samples were first mounted on brass stubs using double sided adhesive tape and then gold coated for 8 min using an ion sputter JEOL, JFC 1100 coating unit. The surface texture of the catalyst was observed using an FEI ESEM Quanta 400. Thermo gravimetric analysis of the catalyst was performed on a thermogravimetric analyzer, TGA-2920, from TA Instruments (USA). Thermograms for the materials were recorded from 30 to 800℃ at a heating rate of 10℃ min- 1 under a nitrogen atmosphere. Bulk density, moisture content, hardness, and ash content were measured using the ASTM test methods D 2854-96, D 2867-95, D 3802-79, and D 2866-94, respectively.
2.3. Fabrication of NBC canister
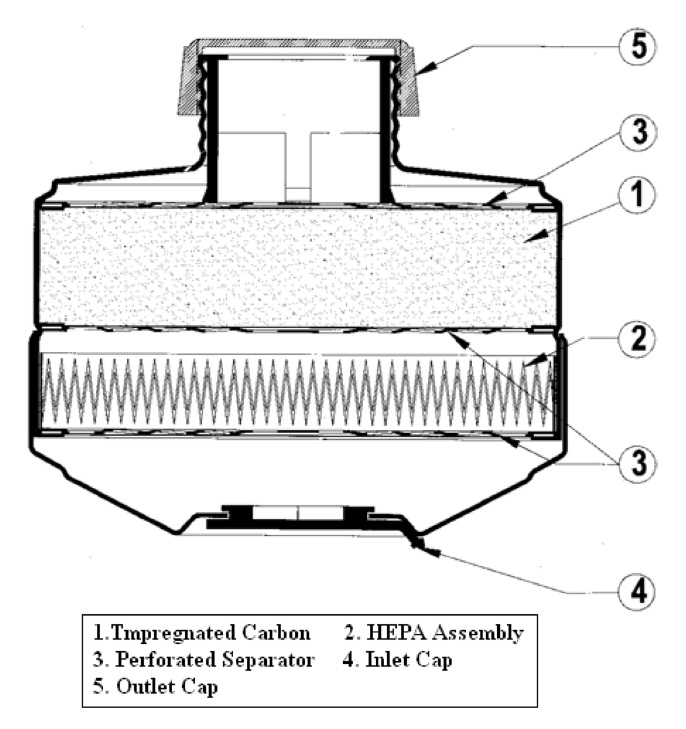
Figs. 1 and 2 show exterior and the cross section of the NBC canister. The NBC canister body (outer part) was made of aluminium and consisted of two main components, i.e., an adsorbent and a particulate filter media. The adsorbent is impregnated carbon (1) and the particulate filter media is HEPA (2), meant for the removal of CW agents and particulates respectively. Adsorbent in an amount of 100 ± 5 gm, having a height of 2.4 ± 0.5 mm, was placed in between the two perforated discs (3); adsorbent was contained and packed with the help of another perforated disc (3) and crimping was done. The HEPA filter media, made up of borosilicate glass fibres, was used in pleated form in the canister to increase the surface area of the filter media for the effective removal of the particulates. This pleated HEPA filter
media was embedded in the assembly. This HEPA assembly (2), having a width of 13 mm, was supported on a perforated disc (3) made up of aluminium. The diameter of the pores in the perforated disc was 5 mm and the thickness of the perforated disc was on the order of 1.0 mm. The perforated disc was wrapped with a nonwoven fabric. The canister consists of threads as per EN 148-1-1987EN, which are fitted to the mask. Inlet (4) allows the air to pass through the canister, exiting by way of outlet (5). The diameter of the inlet and outlet of the canister was 28 mm.
2.4. CCl4 vapour breakthrough measurements of NBC canisters
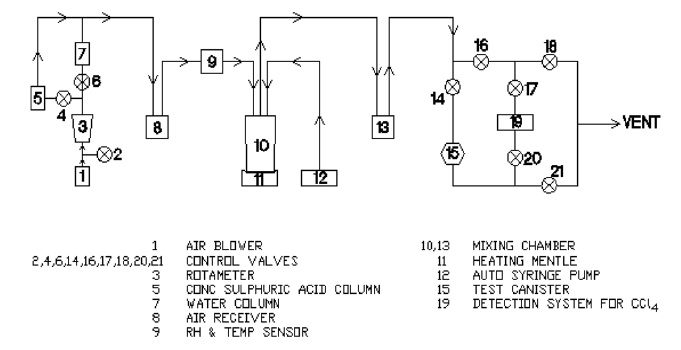
The schematic test setup for CCl4 vapour breakthrough measurements of NBC canisters is shown in Fig. 3. A blower was
used as an air source and air flow was controlled by a rotameter. Relative humidity (RH) was controlled by passing the air through concentrated a sulphuric acid chamber and water chamber. The required concentration of CCl4 vapour was generated by injecting a controlled amount of CCl4 using an auto syringe pump into the mixing chamber, which was maintained at 80℃. CCl4 vapour breakthrough time and breakthrough concentration (1 ppm) were monitored at the outlet of the test canister with the VOC monitor RAE 3000 equipped with a photo ionization detector of 11.7 eV. NBC canisters were evaluated at 30 lpm flow rate of CCl4--air mixture containing 53.13 mg/L CCl4 concentration, at 30 ± 2℃ temperature and 75 ± 5% RH. NBC canisters were also evaluated by varying the CCl4 concentration, test flow rate, temperature, and RH from 5.314-212.52 mg/L, 20-35 lpm, 20-60℃ and 20 to 80%, respectively.
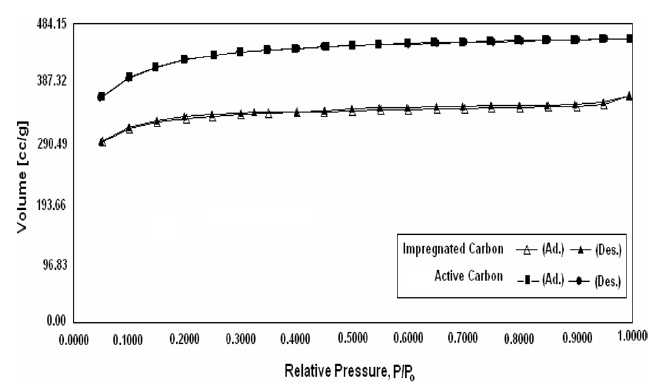
Fig. 4 shows the nitrogen adsorption desorption isotherms of active carbon and impregnated carbon; these isotherms indicate that active carbon as well as the impregnated carbon are highly microporous. The only difference between them upon nitrogen adsorption is the value of the saturation plateau obtained at high relative pressures, which indicates different micropore and mesopore volumes [13]. The surface area of active carbon decreased
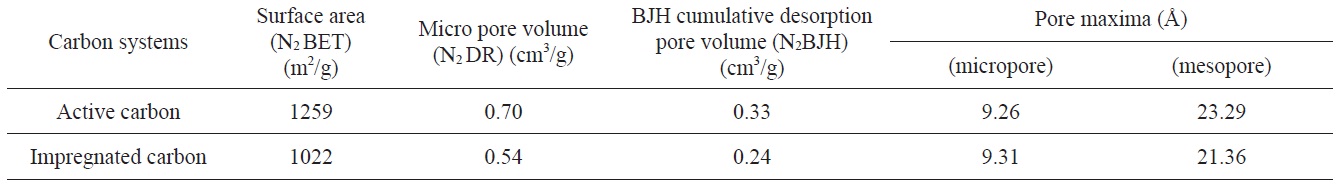
[Table 1.] Surface area and micro pore volume details of the active carbon and impregnated carbon
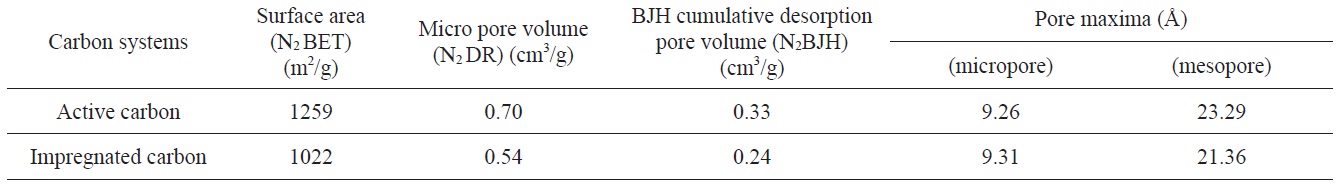
Surface area and micro pore volume details of the active carbon and impregnated carbon
after impregnation from 1259 to 1022 m2/g. This decrease was due to impregnants, which during impregnation travel through the macro pores and get deposited in the mesopores or the pore openings of micropores, hence causing the blockage of the meso and micro pores. The N2 DR micropore volume and N2 BJH cumulative desorption pore volume also indicated the decrease in
micropore and mesopore volume, as shown in Table 1.
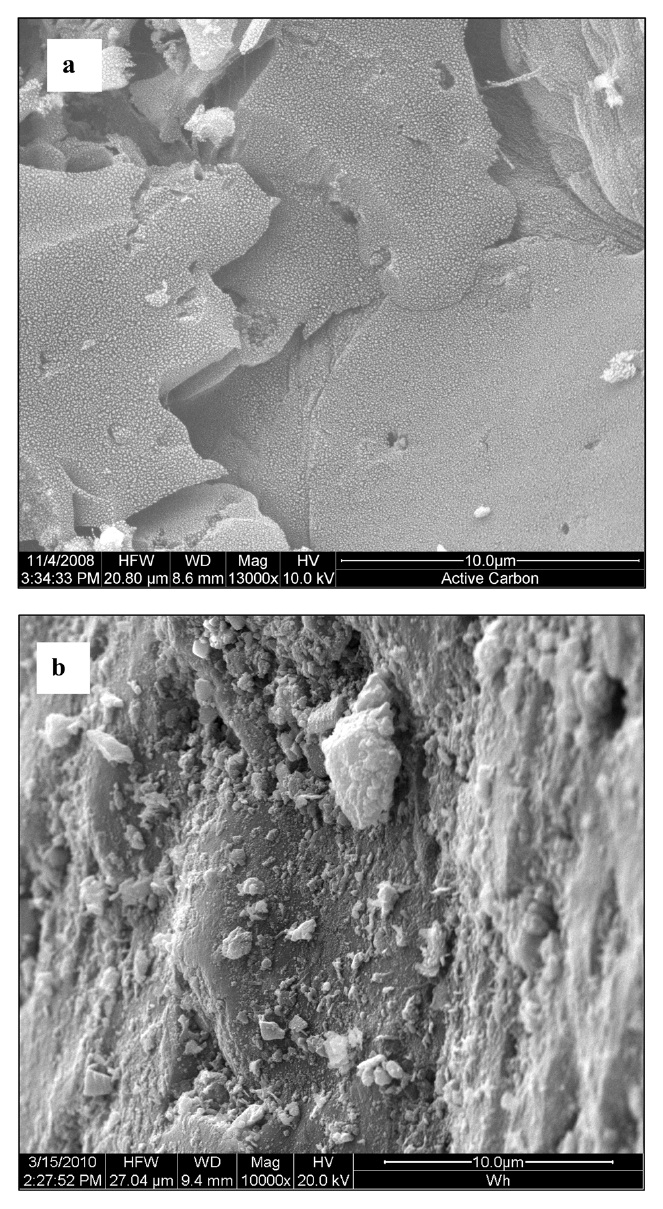
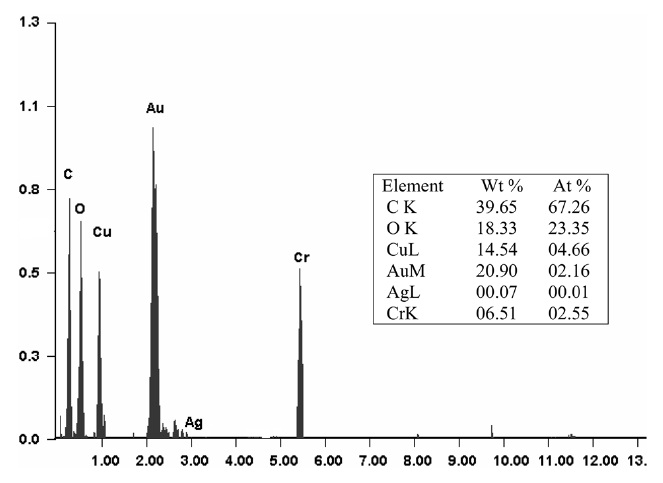
The SEM images of active carbon and impregnated carbon are shown in Figs. 5a and b. One SEM image of active carbon shows considerable small cavities, cracks and attached fine particles over the surface, forming a system of complicated pore networks (Fig. 5a). Impregnation of metal salts leads to the clogging of the cracks and cavities by the adsorbed metal salts and dispersal of metal salts on the surface of the impregnated carbon (Fig. 5b). Another SEM image of impregnated carbon clearly shows the metal salt particles as white spots. The energy dispersive X-ray spectroscopy (EDX) spectra of the impregnated carbon are shown in Fig. 6. The EDX spectra of the impregnated carbon confirm the presence of Cu, Cr, and Ag.
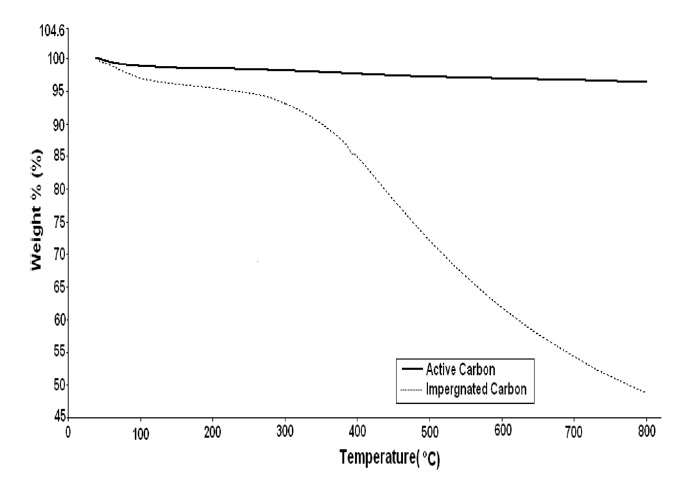
Fig. 7 illustrates the weight change as a function of temperature for active carbon and impregnated carbon in a nitrogen atmosphere. The weight loss as a function of temperature was monitored and used to understand the effect(s) of the metal impregnants on the deterioration of the impregnated carbon. Adsorbed moisture starts getting removed at 110℃. Fig. 7 shows weight losses of about 1.5 and 3.0% in active carbon and impregnated carbon, respectively, at a temperature of about 110℃ because of the loss of adsorbed water molecules (moisture). Impregnated carbon exhibited a steady rate of weight loss up to 280℃. About 7% weight loss took place between 280-380℃. At temperatures higher than 380℃, a marked increase in weight loss was observed up to 800℃. Ehrburger et al. [14] have reported similar results for chromium impregnated carbon. This exotherm is believed to be due to the oxidation of the carbon matrix by the impregnant species. This hypothesis is supported by the behaviour of the active carbon in locations where there are
no impregnants available to oxidize the carbon. Active carbon and impregnated carbon have bulk densities of 0.32 and 0.50 g/ mL respectively. The bulk density of impregnated carbon was found to be higher than that of the active carbon because pores in the impregnated carbon were occupied by impregnants, i.e., the weight increases, whereas the outer surface volume of the material remains the same, and hence the density increases. The moisture content levels of active carbon and impregnated carbon were found to be 5.0 and 1.0%, respectively. The hardness values of both the active carbon and the impregnated carbon were found to be greater than 95%.
3.2. Breakthrough behaviour of NBC canister against CCl4 vapours
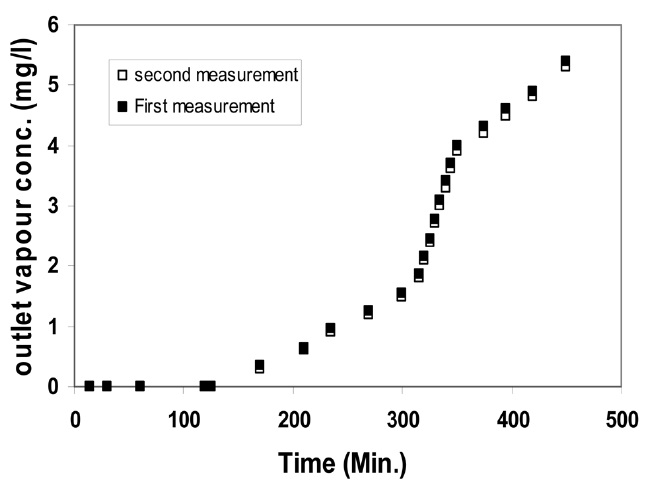
The stability of the CCl4 vapour concentration in the test airflow was monitored before evaluation of the NBC canisters. The coefficient of variation (CV) of CCl4 vapours at different concentrations was found to be within the same range, from 1 to 1.2%. High reproducibility of the CCl4 vapour concentration in the test airflow was also indicated by the breakthrough curves obtained by duplicated measurements, shown in Fig. 8; these
measurements agreed well with each other. This means that the apparatus generated a stable concentration. The breakthrough times of the cartridges or canisters are defined, on principle, as the time when the vapour begins to leak from the test piece at the maximum permissible concentration for the wearers; however, this value is conventionally set at 1 ppm in this study.
3.3. Effect of CCl4 concentration, test flow rate, and temperature
It was observed that the breakthrough time of the NBC canister decreases with the increase of the vapour concentration of CCl4 [15]. At inlet concentrations of 13.28, 26.56, 53.13, 106.26, 159.39, and 212.52 mg/L the breakthrough times were observed to be 82, 39, 19, 10.5, 7, and 5.5 min, respectively, at 30 lpm flow rate. It was also observed that with the increase of the test flow rate and temperature, the breakthrough time of the NBC canister decreased. At inlet flow rates of 20, 25, 30, and 35 lpm the NBC canister showed breakthrough times of 15, 13, 10.5, and 7 min, respectively, at the inlet concentration of 53.13 mg/L. With increasing flow rate, the breakthrough time decreases due to there being less contact time [2]. The contact time decreases from 0.56 to 0.32 s when the flow rate increases from 20 to 35 lpm. In the temperature range of 20-40℃, the NBC canister showed a breakthrough time of 19 min, whereas the increase of temperature from 40 to 60℃ resulted in a decrease of the breakthrough time to 18 min. This may be attributed to the decrease in adsorption of CCl4 vapours at higher temperature [2].
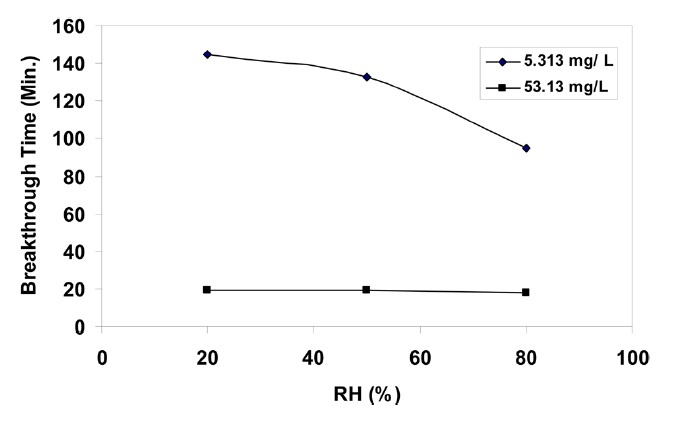
3.4. Effect of relative humidity
The performance of the NBC canister against a CCl4 -air mixture having 53.13 and 5.313 mg/L concentrations at different RH under the aforesaid experimental conditions is presented in Fig 9. The figure indicates that the NBC canister does not show much variation in the breakthrough behaviour of CCl4 vapour in the range of 20 to 80% RH at 53.13 mg/L, due to the lowered susceptibility of the NBC canister with coexisting moisture. This can be attributed to the fact that higher concentrations of CCl4 vapour cause a stronger exothermic reaction, which results in an increase of the temperature of the adsorbent. This sudden increase of temperature nullifies the effect of high humidity. The breakthrough behaviour of the NBC canister at 5.313 mg/L indicates that at lower CCl4 vapour concentrations RH affects the breakthrough time. An increase of RH from 20 to 80% at 5.313 mg/L results in a decrease of the breakthrough time from 145 to 95 min. This apparent difference in the breakthrough behaviour of the NBC canister against CCl4 vapour at high RH can be interpreted through the idea that CCl4 and H2O molecules adsorb competitively on carbon, which in turn results in a blockage of active sites of the carbon by H2O adsorption [16].
The breakthrough behaviour of an indigenously developed NBC canister for CCl4 vapours was studied and interpreted in terms of the CCl4 BTT value. The effects of various parameters such as CCl4 vapour concentration, test flow rate, temperature, and RH were also studied. The results showed that CCl4 BTT values were observed to decrease with the increase of CCl4 concentration and inlet flow rate. The variation in temperature and RH did not have much effect on the CCl4 breakthrough behaviour of the NBC canister at high vapour concentration of CCl4, whereas the breakthrough times of the NBC canister were shortened by an increase of RH at low CCl4 vapour concentration. Therefore, it can be concluded that the indigenously developed NBC canister, having Cu, Cr, and Ag impregnated carbon, is a promising device that can be used for protection against deadly toxic CW agents.