The discovery of graphene has been one of the most outstanding recent achievements [1,2]. Graphene is one of the most promising materials in nanotechnology because of its exceptional physical properties, such as high electronic conductivity, good thermal stability, and excellent mechanical strength. In particular, the promising properties together with the ease of processibility and functionalization make graphene-based materials ideal candidates for incorporation into various functional groups or materials.
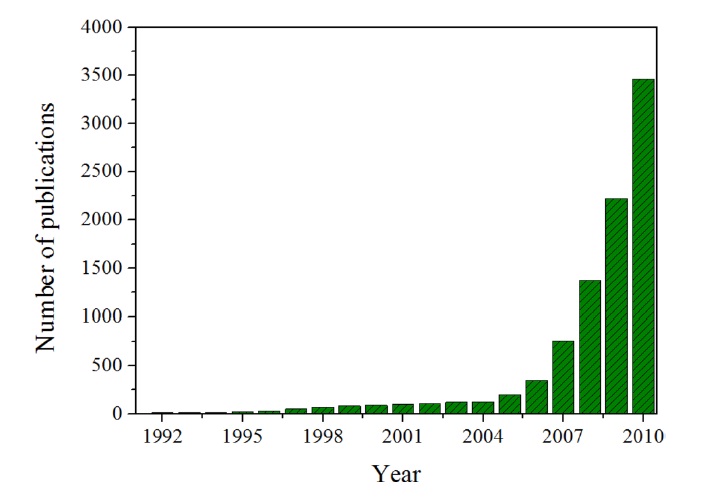
As Fig. 1 demonstrates, the optimism about graphene is validated by the number of publications related to graphene, which dramatically increased after 2004 [3].
Importantly, graphene and its derivatives have been explored for a wide range of applications
in such fields as electronic and photonic devices, clean energy, and sensors [4-6]. Other forms of graphene-related materials, including graphene oxide, reduced graphene oxide, and exfoliated graphite, have been reliably produced on a large scale from the mother of all graphitic materials, as presented in Fig. 2 [7]. Graphene, one of the allotropes (such as carbon nanotubes, fullerene, diamond, and so on) of elemental carbon, is a planar monolayer of carbon atoms arranged in a two-dimensional (2D) honeycomb lattice with a C-C bond length of 0.142 nm [8]. Graphene is the thinnest of known materials in the universe and the strongest ever measured.
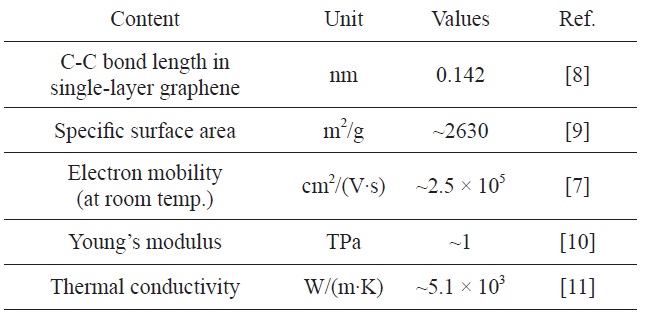
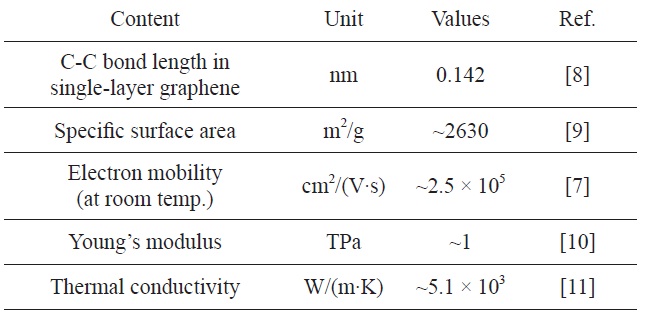
Its extended hexagonal monolayer network is the basic building block of other important allotropes, which can be stacked to form 3D graphite, rolled to form 1D nanotubes, and wrapped to form 0D fullerenes. As can be seen in Table 1, it has demonstrated a variety of intriguing properties, including high electron mobility at room temperature (250 000 cm2/[V·s]) [7], high specific surface area [8], superior mechanical properties with Young’s modulus of 1 TPa [10], and exceptional thermal conductivity (5000 W/[m·K]) [11].
These potential characteristics are applied to single molecule gas detection, transparent conducting electrodes, composites, and energy storage devices, such as supercapacitors and lithium ion batteries [12-16]. Furthermore, a distinct band gap can be formed as the dimensions of graphene are reduced to narrow ribbons with a width of 1-2 nm, producing semi-conductive graphene with potential applications in transistors [17].
More recently, the adsorption behavior of graphene and graphene- based materials (its derivatives) has also been of interest due to graphene’s high specific surface area and newly created textural properties.
After a general introduction to graphene and its derivatives, this review will demonstrate that graphene is not confined to only single-layer graphene nanosheets; it can have two-, three-, or multi-layer nanosheets. This will even include the graphenebased materials (its derivatives). Because adsorption behavior is mainly studied with graphene and its derivative-based materials, the purpose of this review is to look at, in a brief way, what is re-
[Table 1.] Physical properties of single-layer graphene at room temperature
Physical properties of single-layer graphene at room temperature
ferred to as graphene in the literature and the adsorption behavior of graphene and graphene-related materials for gas sensors, hydrogen storage, carbon dioxide capture, and so on.
Graphene with varying number of layers can be synthesized using different strategies, such as the growth of carbon nanotubes producing graphite with 100 layers of graphene, chemical vapor deposition in metal surfaces (a few layers of graphene), the thermal decomposition of SiC, micromechanical exfoliation cleavage, chemical reduction of graphene oxide, and so on. Although, these various approaches did not produce perfect monolayer graphene, some studies showed that the chemical vapor deposition method has been optimized and become a major technique to produce graphene in large quantities [18]. Now, we briefly overview the various synthetic methods and the properties of the graphene produced by each synthetic method.
2.2. Epitaxial growth and chemical vapor deposition technique
For the development of large-scale synthesis of grown graphene, several methods have been studied by many researchers, including graphitization of SiC surfaces [19,20] and chemical vapor deposition (CVD) on transition metals [21-24].
At first, epitaxial graphene research evolved out of work on carbon nanotubes. It is well known the carbon nanotubes have superior electronic properties. However, the inability to manufacture well-controlled tubes and scale them up from singletube transistors to large-scale integrated circuits has prevented expanded technological applications. It is presented in Fig. 3.
De Heer et al. [25,26] realized that two-dimensional graphene, essentially unrolled nanotubes, would have many of the same properties as carbon nanotubes, and this idea opened the way to a new approach to carbon electronics. With an epitaxial method, ultrathin epitaxial graphite was grown on single-crystal silicon carbide by vacuum graphitization at high temperature. The material can be patterned using standard nanolithography methods [24-26].
Recently, Bae et al. [27] reported a roll-to-roll production of 30-inch graphene films using the CVD method. Their fabrica
tion process including three steps after the synthesis of graphene on copper substrates: (i) adhesion of polymer supports to the graphene on the copper foil; (ii) etching of the copper layers; and (iii) release of the graphene layers and transfer onto a target substrate (Fig. 4).
Epitaxially grown graphite has a number of differences related to physical properties in comparison with mechanically exfoliated graphene [28] due to the influence of interfacial effects in epitaxial graphene, which are strongly dependant on both the silicon carbide substrate and some growth parameters.
The second method involves substrate-based growth of single layers by a CVD technique. It has been reported that large and predominantly monolayer graphene of excellent quality can be synthesized by CVD on polycrystalline Ni, Cu, Co, Ru, and other transition metals [29,30], with the flow of a hydrocarbon gas, such as methane, ethylene, acetylene, and benzene. In this procedure, the substrate is subjected to electron bombardment in an ultrahigh vacuum at 1000℃ to remove oxide contaminants and then heated to temperatures ranging from 1250℃ to 1450℃ for a few minutes. Blakely et al. [31,32] reported the formation of carbon films by the cooling of Ni foils saturated with carbon at high temperatures.
This is known to be relatively simple and economical, and has been used to produce graphene that can reach impressive sizes and can be easily transferred to other substrates [33,34].
Both epitaxial synthesis and CVD techniques take advantage of specially chosen platforms to encourage high-quality growth. They also have the prospect of producing a single sheet of graphene over an entire wafer.
Meanwhile, the following solution-based synthetic schemes by chemical reduction or exfoliation have no need for support substrates.
2.3. Micromechanical exfoliation technique
The micromechanical exfoliation technique is a simple peeling process. Fig. 5 show how a commercially available highly oriented pyrolytic graphite sheet was dry etched in oxygen plasma into many 5 μm deep mesas.
Novoselov et al. [1] first observed single-layer graphene in 2004, as presented in Fig. 6. They isolated two-dimensional crystals from three-dimensional graphite using the mechanical exfoliation technique. As a result, single- and few-layer flakes are locked up substanding by only van der Waals forces and can be made free-standing by etching the substrate [35-38]. This attracted tremendous attention.
To exfoliate a single layer using mechanical exfoliation, the key is to overcome the van der Waals attraction [39,40] between exactly the first and second layers without disarranging any subsequent layers. However, this method is a typical top-down approach of graphene, which requires manual effort and produces unreliable results, while small areas hinder practical applications of graphene [41,42].
Meanwhile, it is interesting note that mechanical exfoliation with cellophane tape from graphite is still considered best for producing the highest performing samples [39], among the various methods.
2.4. Chemical reduction and thermal exfoliation
In 1958, Hummers and Offeman [43] reported a method of preparing graphene oxide, which readily forms a stable colloidal
suspension in water. This graphene oxide suspension is used to facilitate production of single-layer graphene oxide by ultrasonic treatment (under the hundreds of W and tens of kHz) [44-46].
By modifying the Hummers’ methods, a tremendous number of studies on the synthetic method of graphene production have been published, and the produced graphene has been applied in a variety of applications. With improvement, gram quantities of graphene can also be obtained by a solvothermal procedure for preparing graphene oxide using sodium, potassium, ethanol, and so on [47,48].
To synthesize graphene, the reduction of graphene oxide by chemical methods has been carried out using reducing agents, such as hydrazine [49-52], dimethylhydrazine [53], hydroquinone [54], and NaBH4 [55,56]. A proposed reaction pathway for epoxide reduction with hydrazine is presented in Fig. 7.
The chemical reduction method by graphene oxide in solu-
tion allows easy manipulation and transfer of graphene onto substrates.
Erickson et al. [57] have investigated the local chemical structures on graphene oxide and reduced graphene oxide using the transmission electron microscopy (TEM) measurement (a monochromated aberration-corrected instrument operated at 80 keV). The TEM image of graphene oxide clearly shows the oxidized area (Figs. 8a and b) and unoxidized graphene crystal area (Fig. 8c). As explained in the figure caption, hydroxyl groups and epoxy were presented on the graphene oxide basal plane. On the other hand, the TEM image of reduced graphene oxide shows the disordered regions (Fig. 8a) that is believed to result from the oxidized area being reduced by hydrazine and thermal annealing, and the unoxidized graphene regions.
An important method to synthesize graphene is thermal exfoliation of graphene oxide at high temperatures [58-60]. In this method, chemically prepared graphene oxide is placed in a well-sealed long-type quartz tube and then purged with inert gas. Then, the tube containing graphene oxide is quickly inserted into a muffle or tubular furnace preheated to 1050℃ and
maintained at that temperature for around 10 min. This is usually called exfoliated graphene.
Kaniyoor et al. [60] investigated that the effect of different exfoliation conditions on the synthesis of graphene from graphite oxide, for example, Ar at 1050℃ , vacuum at 200℃ , H2 + Ar mixture at 200℃ , and H2 at 200℃. They found that these results showed conclusively that the atmosphere for exfoliation of graphite oxide plays a critical role in low temperature synthesis of graphene. It was observed that exfoliationreduction of graphite oxide in pure hydrogen atmosphere at 200℃ results in the highest quality of a few layered graphene sheets. These schematic representation of the mechanisms are presented in Fig. 9.
Few-layer graphene is synthesized by microwave treatment, conversion of nanodiamonds, and arc discharge of graphite. The method of preparing graphene by microwave treatment is just a post-process using chemically synthesized graphene oxide in large quantities in a short time [61-63]. Andersson et al. [64] and Prasad et al. [65] reported that graphene can be synthesized by heating nanodiamonds in an inert or reducing atmosphere. Nanodiamond particles are treated by soaking them in concentrated hydrochloric acid to avoid contamination by magnetic impurities. Then, they are annealed in a graphite container in a graphite furnace in a helium atmosphere at temperatures ranging from 1600 to 2500℃.
Subrahmanyam et al. [66] reported that arc discharge of graphite in a hydrogen atmosphere yields graphenes exclusively containing 2 to 3 layers, although the flakes are relatively small (100 to 200 nm). It has been found that this process depends on the presence of hydrogen gas during the arc discharge which terminates the dangling carbon bonds with hydrogen, which prevents the formation of closed structures [67]. To prepare graphene by the arc discharge method, direct current arc discharge of graphite evaporation is carried out in a water-cooled stainless steel chamber filled with a mixture of hydrogen and helium without any catalyst [68]. It has been reported that the best transition characteristics are exhibited by graphene synthesized by arc discharge of graphite in hydrogen.
The unique chemical properties of graphene affecting its adsorption behavior have only recently attracted the attention of many researchers; thus, it has not yet been studied in sufficient detail. Therefore, we will attempt to elucidate the adsorption behaviors of graphene and graphene-based materials.
Adsorption behavior depends significantly on the unique structure and concentration of chemical species created by a process of preparing graphene. It is possible to possess a large high surface area, sufficient porosity, superior conductivity, broad potential window, and rich surface chemistry. In addition, the extended polyaromatic π-electron system and coordinatively unsaturated terminal carbon atoms have a decisive effect on the reactivity of graphene. The readily polarizable π-electron system of graphene is equally reactive with electro- and nucleophilic species, and this makes the system sensitive even to radical species.
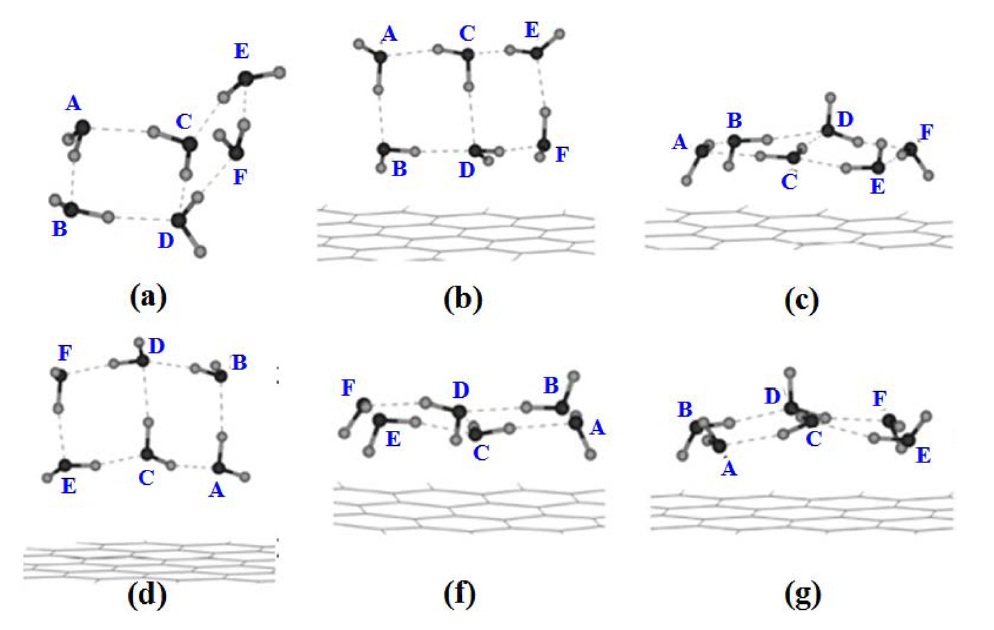
A water cluster adsorbed on a graphite surface is a prototypical weakly bound van der Waals π-system that involves watergraphite and water-water interactions. Zhang and Sarkar [69] investigated that the binding energy of water clusters interacting with graphite is dependent on the number of water molecules that form hydrogen bonds, but is independent of the water cluster size, as presented in Fig. 10.
Furthermore, Lin et al. [70] found that these physically adsorbed or physisorbed water clusters show little change in their IR peak position and leave an almost perfect planar graphite surface. This is shown in Fig. 11.
The US Department of Energy’s revised gravimetric capacity target for hydrogen storage systems for 2015 is 5.5 wt%. These targets have not yet been reproducibly met by any available storage technology. Solid carbon adsorbents, such activated carbons [71,72], fullerenes [73,74], carbon nanotubes [75-78], graphites, carbon nanofibers [79-81], ordered porous carbon [82,83], and carbon-based hybrid composites [84,85], and so on, had previously been considered to be promising candidates for hydrogen storage due to their large surface area, structural stability, and light mass. In particular, the adsorption mode of H2 on carbon nanomaterials is reversible weak physisorption, which is a key factor in the operation of hydrogen/fuel-cell-powered systems.
To satisfy the increasing need for hydrogen storage, graphene has been attracting much attention as the newest carbon materials. It appears to be composed of a monolayer of carbon atoms packed into a dense honeycomb crystal structure. Single-layer graphene is theoretically predicted to have a large surface area of 2600 m2/g, while the specific surface area of few-layer graphenes prepared by various methods is in the range from 250 to 1500 m2/g.
Furthermore, recently, graphene-like nanosheets (in some cases, it has contained some oxygen functionalities; therefore,
all of these graphene-like nano-materials are called “graphene oxide”) have been studied in relation to the adsorption of hydrogen. They are produced relatively easily by oxidative treatment of graphitic materials with strong oxidizing agents. Graphene oxide is a stacked structure similar to graphite but with a wider spacing between graphene oxide nanosheet layers with diverse range of 6 to 15 A [86].
Srinivas et al. [87] studied graphene oxide prepared by the reduction of a colloidal suspension of exfoliated graphite oxide for hydrogen storage capacity. The nitrogen/77 K adsorptiondesorption isotherms and TEM/SEM images of synthesized
graphene oxide are presented in Figs. 12 and 13, respectively. As can be seen in Figs. 14 and 15, the sample was found to be in a highly agglomerated state with many wrinkles, and it had a specific surface area of 640 m2/g as measured by nitrogen adsorption at 77 K.
The isotherms exhibited a typical type-I curve at low relative pressure and a hysteresis loop at relative pressure from 0.4, indicating the presence of microporosity, mesoporosity, and some macroporosity [88]. They explained that the overlap and stacking of the exfoliated layers influenced the significant low textural characteristic.
In the Srinivas study, it was shown that the highest hydrogen storage capacities of a prepared sample were about 1.2 wt% and 0.1 wt% at pressures up to 10 bar under the temperatures
of 77 K and 298 K, respectively. Furthermore, they predicted the adsorption capacities at 100 bar of ~3 wt% and 0.72 wt% at 77 K and 298 K, respectively, obtained by linear extrapolation using the model LangmuirEXT2 expression. The room temperature hydrogen adsorption capacity was higher than other recent experimental results on hydrogen storage in a pristine graphene sample [89], which might be attributable to the higher isosteric heat of adsorption values. Ma et al. [89] reported that 0.4 wt% and ~0.2 wt% hydrogen uptake of graphene oxide were obtained at 77 K under 1 bar and 298 K under 60 bar, respectively.
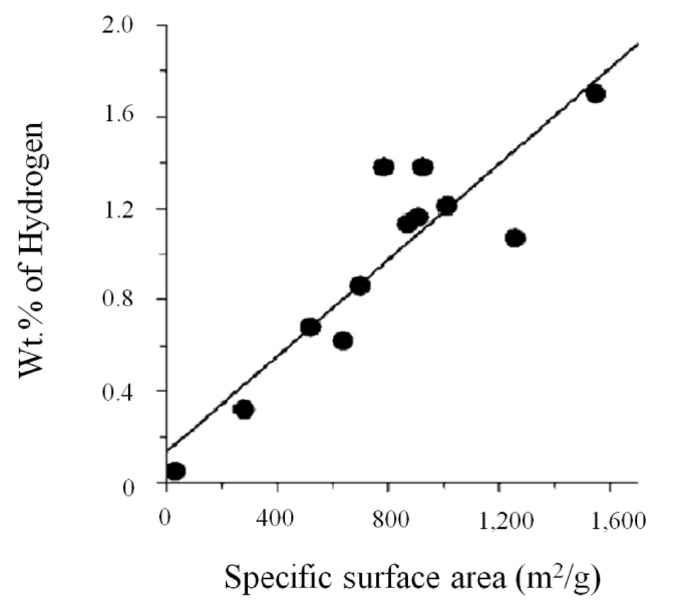
Ghosh et al. [90] found that high hydrogen adsorption capacity on graphene oxide obtained by thermal exfoliation or conversion of nanodiamond was 3.1 wt% (in case of single-layer graphene) at 298 K and 100 bar. As shown in Fig. 14, the hydrogen uptake varied linearly with the specific surface area, and the presence of more than one layer of graphene oxide in this study caused a decrease in the hydrogen uptake. In addition, the specific surface area of graphene oxide depends on the number of layers, not the pore effect [91,92].
It is interesting to note that the H2-graphene interaction energies obtained in their study satisfy the optimum conditions for adsorptive storage of hydrogen [93]. They also expected that higher values for hydrogen storage should be possible by preparing better samples and by reducing the average number of graphene layers.
The transition metal loaded/graphene oxide composites for hydrogen storage are being extensively researched. Wang et al. [94] have investigated the structural properties of graphene oxide and the feasibility of using graphene oxide to anchor Ti for hydrogen storage with first-principles computations. They reported that on graphene oxide there exist stable structural motifs as the basic function units of oxygen functionalities. It is consid-
ered that graphene oxide could be an excellent substrate for Ti, but for hydrogen storage, it requires the hydrogenation of epoxy and open sp2 sites. Upon hydrogenated graphene oxide, the Ti binding energy is increased to a level that is high enough to prevent Ti clustering. Dihydrogen binding energy is also favorable for room temperature storage. The theoretical gravimetric density was 4.9 wt%. Thus, Ti-anchored graphene oxide may offer a feasible solution for hydrogen storage.
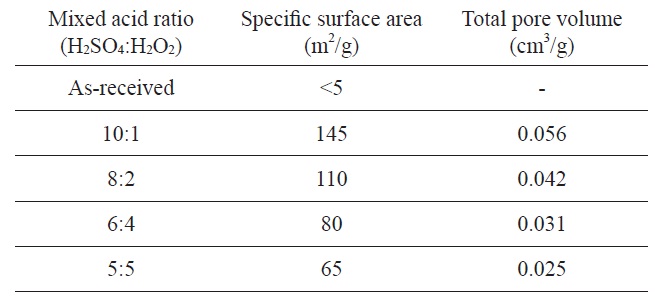
Kim and Park [95] have reported nickel/graphite hybrid materials which are produced by mixed acid treatment of graphite flakes for hydrogen storage. The graphene oxide samples were prepared by dipping graphite flake into a mixed acid solution (H2SO4 and H2O2) in a range of from 10:1 to 5:5 at room temperature. The experimental conditions of mixed acid treatments and textural properties of produced graphene oxides are listed in Table 2.
Fig. 16 is a schematic of the pore development of a graphite flake using the acidic method. The layer distance of each graphite sheet is nearly 0.354 nm, and it is impossible for hydrogen molecules to be adsorbed at this dimension. However, after the expansion of the layers, the layer distance widens and new adsorption sites become exposed for hydrogen molecules.
Furthermore, Ni nanoparticles were loaded onto graphene ox-
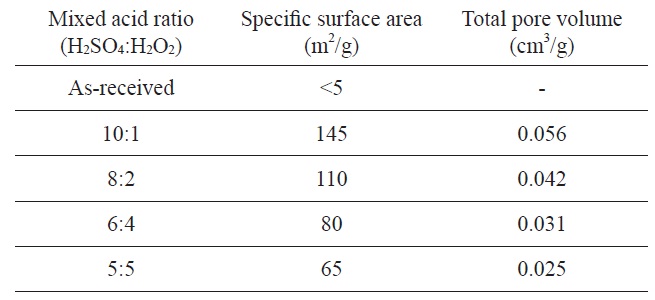
Specific surface area and pore volumes of graphene oxide as a function of the mixed acid ratio
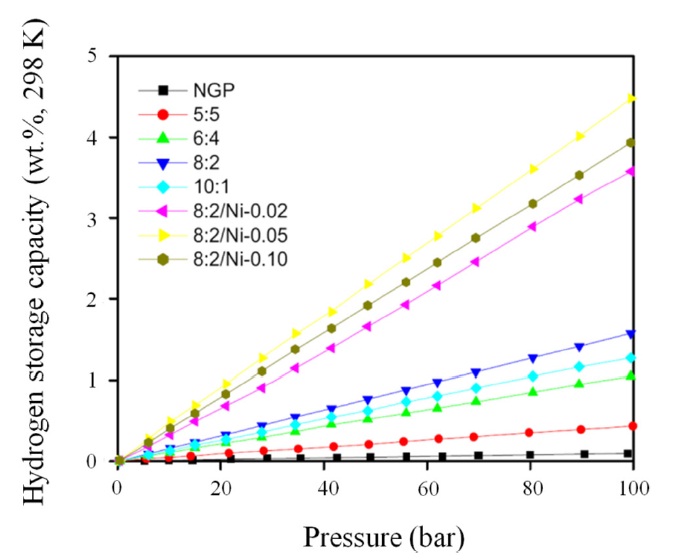
ide surfaces as a function of Ni content in an effort to increase the final hydrogen uptake. In Fig. 17, the highest hydrogen storage result of the 8:2/Ni-0.05 sample was 4.48 wt%, which was prepared by 0.05 wt% of NiSO4 per 1 g of graphene oxide.
However, it is important to note that the 10:1 sample showed lower hydrogen uptake the 8:2 sample, though the former had a higher specific surface area. This result indicates that the 8:2 samples may have a more suitable pore dimension for the adsorption of hydrogen molecules. Though the 10:1 sample had a high specific surface area, it was checked by a nitrogen molecule, indicating that the area was not suitable for hydrogen molecules.
Park et al. [95] suggested the newly a dipole-induced mechanism for hydrogen storage. As shown in Fig. 18, the first hydrogen molecules introduced can be reacted with metal particles due to the high oxidation power of metals on graphite surfaces due to the Kubas reaction [96,97].
Here, unlike the chemical binding of hydrogen atoms to transition metal on carbon surfaces, lone transition metal atoms bind hydrogen via the so-called “Kubas interaction,” in which the
H-H bond undergoes some elongation rather than complete dissociation into atoms.
However, the amount of this initial reaction is very small. The binding energy and bond distance in this case are 85 kJ/mol and 0.2 nm, respectively, because the binding energy and bond distance of metal hydride are normally close to 85 kJ/mol and 0.2 nm, respectively. The second group of hydrogen molecules introduced cannot chemically react due to a site limitation caused by the initially introduced hydrogen molecules; however, they are physically adsorbed by dipole-induced effects. The hydro-
gen molecules are basically non-polar, but the strong interaction of the metal particles leads to the dipole inducing effects of the hydrogen molecules. The third group and any remaining hydrogen molecules are adsorbed by the same mechanism of high gas pressure, but this equilibrium can be broken when the interaction of oxidized graphite surfaces and dipole-induced hydrogen molecules is stronger than metal-hydrogen interactions [98-102]. This mechanism can be applied only under higher pressure, and when graphite supports are charged strong electron acceptors.
These unique characteristics of graphene oxide can play a leading part in enhancing hydrogen storage capacity.
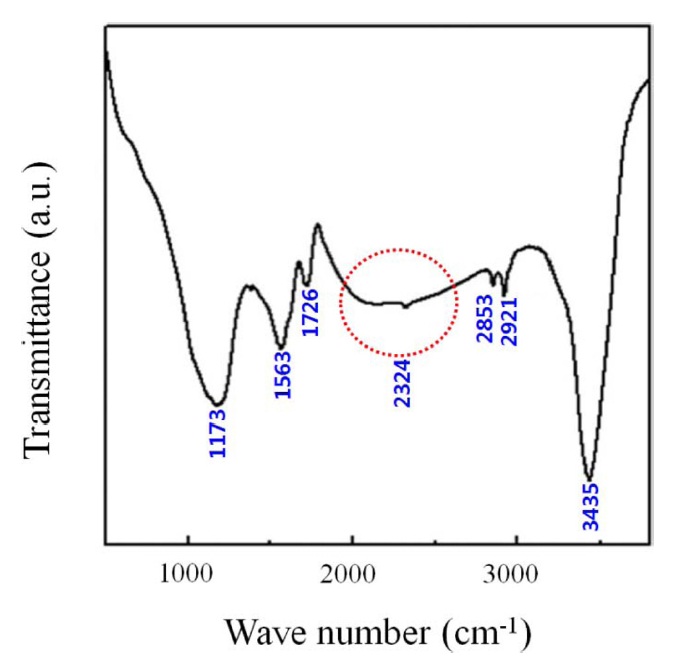
Mishra and Ramaprabhu [103] suggested graphene oxide for CO2 adsorption, which is prepared by hydrogen-induced thermal exfoliation of graphene oxide at 200℃ for possible large-scale production of graphene [104]. They confirmed the physical adsorption of CO2 in graphene oxide using Fourier transform infrared spectroscopy.
In Fig. 19, the band corresponding to ?OH groups at 3435 cm-1 is quite prominent compared by the insignificant ratios of
anti-symmetric and symmetric =CH2 vibrations for graphene oxide in this work. Furthermore, the intense peaks correspond to the carboxylic and carbonyl groups at 1726 (>C=C), 1563 (=CH-), and 1173 cm-1 (>C=O). An additional peak of 2324 cm-1 corresponds to the adsorbed molecular CO2 in graphene oxide resulting from physical CO2 adsorption. They reported that the maximum adsorption capacity was 21.6 mmol/g at 298 K and 11 bar. This value is higher than that of other adsorbents. In addition, they compared the CO2 adsorption capacity of graphene oxide with those of other usual adsorbents, such as, carbon materials, MOF, zeolite, and so on, as mentioned below.
Before everything else, Mishra and Ramaprabhu [105] reported CO2 adsorption capacity, obtained by study of multiwalled carbon nanotubes, was observed to be 11.7, 8.3, and 7.0 mmol/g at 11 bar and 25℃ , 50℃, and 100℃, respectively. Also, Siriwardane et al. [106] and Gensterblum et al. [107] reported 7 mmol/g and 6 mmol/g, respectively, of CO2 adsorption in activated carbon at 45℃ and 11 bar. Zhang et al. [108] reported enhancement of around 20% in CO2 uptake, which was achieved by modifying the AC with nitrogen at 298 K, and adsorption capacity was about 16 mmol/g adsorption capacity at 11 bar. Cavenati et al. [109] reported 3.2 mmol/g of CO2 adsorption in 13X zeolite at 298 K and 12 bar. A high-pressure CO2 adsorption study on various metal organic frameworks by Millward and Yaghi [110] achieved CO2 adsorption capacity ranging from 2 to 8 mmol/g under similar conditions.
In addition, Wang et al. [94] reported CO2 adsorption by graphene oxide. They studied the CO2 adsorption of graphene oxide at 195 K and 1 bar, which conditions showed somewhat variable values of CO2 uptake in the range from 10 to 38 wt%. It is possible that low CO2 uptake values can occur in graphene samples containing large particulates.
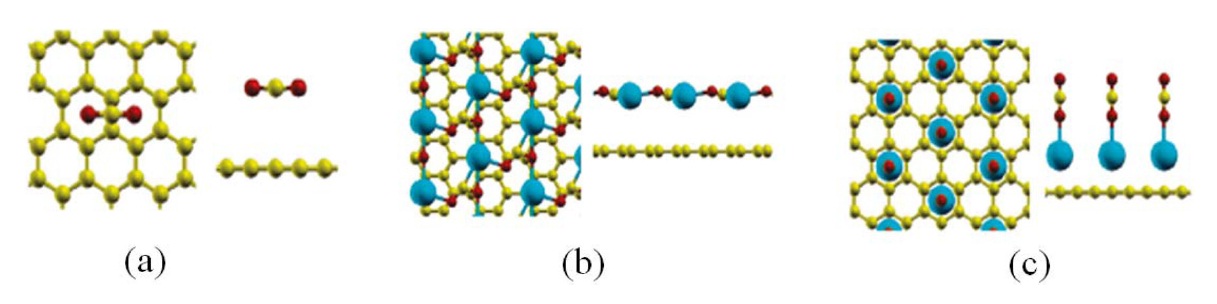
Next, we will discuss metal-loaded graphene composites for CO2 adsorption. As shown in Fig. 20, Cazorla et al. [111] performed a first-principles study of CO2 adsorbent materials consisting of calcium atoms and carbon-based nanostructures.
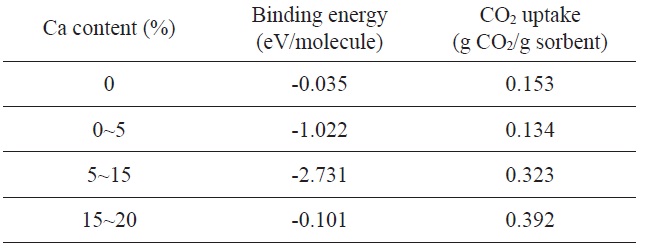
They found that Ca-decorated graphene possesses unusually large CO2 uptake capacities in the range from 0.1 to 0.4 g CO2/g sorbent under a low-gas-pressure regime as a result of their unique topology and a strong interaction between the metal nanoparticles and CO2 molecules. It is also reported that the strength of the Ca metal and CO2 molecule interactions can be efficiently tuned as a function of the Ca loading content (Table 3).
Enhancement of the CO2 reactivity properties of carbons in the presence of Ca atoms can be understood in terms of electronic structure, that is, overlapping of s,d-metallic and p-molecular states in the region near the Fermi level. Thus, by use
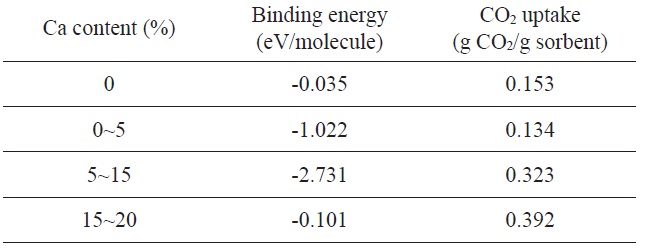
Binding energy (per CO2 molecule) and corresponding gas-adsorption capacities of Ca/graphene as a function of Ca content
of first-principles simulation techniques, Guo et al. [111] confidently proposed that these Ca metal/carbon-based nanomaterial composites exhibit characteristics of suitable adsorbents for CO2 capture and sequestration applications.
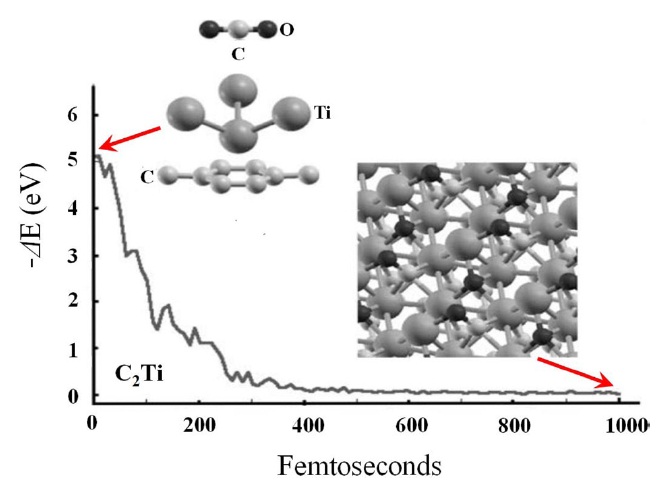
Carrillo et al. [112] studied the adsorption of CO2 on a Tigraphene system with high metal coverage using the density functional theory and molecular dynamics simulation. Positively charged Ti atoms on graphene surfaces attract negatively charged oxygen atoms towards the graphene surfaces. This force is stronger than the initial repulsion on the C atom by the Ti atoms. When a CO2 molecule approaches the graphene surfaces, the CO2 molecule cannot be linear any longer. The O atoms in a bent CO2 molecule are under different force fields. Thus, one O atom traps an electronic charge from the Ti atoms of the upper plane and ends bonded to four Ti atoms, resulting in the CO2 molecule interacting very strongly with the Ti atoms of the upper plane, as can be seen in Fig. 21.
To complete the discussion of Carrillo et al. [112] mentioned above, it is essential to place mono-dispersed Ti atoms on a graphene surface. Ma and Resenberg [113] reported that their experiments showed that adsorbed Ti atoms on a clean graphite surface tend to form islands or some clusters. Meanwhile, Zhang et al. [114] showed that not only the Ti coating atoms can be dispersed along single-walled carbon nanotubes; Al and Au nanoparticles are also uniformly deposited on the pre-treated nanotubes, which means that this procedure could facilitate the dispersion of Ti atoms on graphene surfaces.
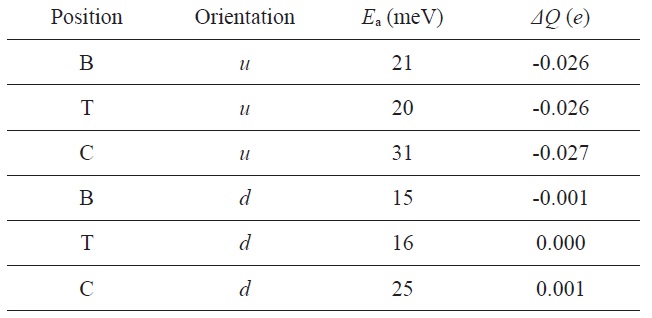
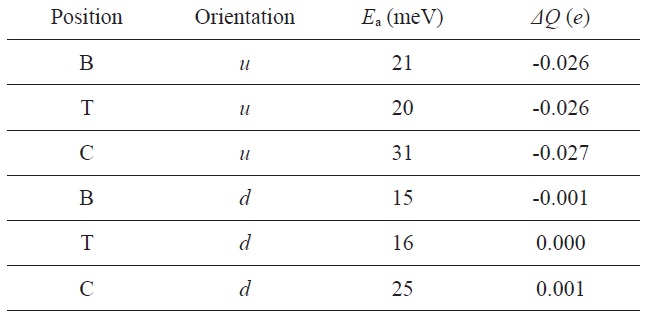
NH3 on graphene: the adsorption energy (Ea) and the charge transfer (ΔQ) from graphene to the molecule for various adsorption sites and orientations
Hugo et al. [115] reported studies of the interaction of NH3 with a graphene field-effect transistor (FETs) supported on Si/ SiO2 substrates to provide further insight into the nature of the molecule-graphene interaction mechanism. Here, SiO2 is used as a gate dielectric, and the heavily doped Si substrate is used as the bottom gate electrode. From the study, they elucidated that graphene FETs initially behave as a p-type system due to exposure to air, while these FETs can be rendered n-type by longterm vacuum-degassing at 200℃ .
Leenaerts et al. [116] investigated the adsorption process of NH3 molecules on graphene through first-principle calculations and showed the presence of two main charge transfer mechanisms. They considered two different orientations of NH3 molecules with respect to the graphene surface: (i) the H atoms pointing away from the surface (
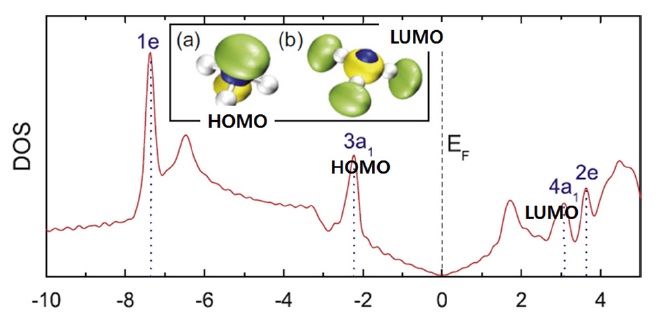
Fig. 22 shows this dependence on the orientation can be understood in terms of the highest occupied and lowest unoccupied molecular orbital (HOMO and LUMO) of the molecule and the total density of states of the system. The HOMO and LUMO are both close enough to the Dirac point to cause some charge transfer through orbital mixing (hybridization) with the graphene orbital. In the u orientation, the NH3 molecule acts as a donor, because the HOMO is the only orbital that can have a significant overlap with the graphene orbitals, and as a consequence, acts as a donor in this orientation. Meanwhile, in the d orientation, both the HOMO and LUMO of the NH3 molecule are able to interact with graphene. This leads to competing charge transfers to and away from graphene, resulting in a total charge transfer close to zero. As observed experimentally in their study, the u orientation is energetically favored, which explains the donor character of NH3 molecules.
The field of graphene-related research has grown at a spectacular pace since a single-layer flake was first isolated in 2004. The Nobel Prize in Physics 2010 was awarded to Andre Geim and Konstantin Novoselov for ground breaking experiments. Graphene and graphene-based materials are promising candidates as components in various applications due to their superior properties. Recently, the development of various methods for producing these materials has stimulated a vast amount of research, for example, epitaxial growth and chemical vapor deposition techniques, micromechanical exfoliation, chemical reduction, and thermal exfoliation, microwave treatments, and conversion of nanodiamonds. A proper understading of the growth and properties of graphene is a must for its optimal utilization.
The main purpose of this review was to comprehensive the synthesis method of graphene and to brief the adsorption behaviors of graphene and graphene-based materials. We have also reported not only the nature of adsorption sites for various gases on their surfaces for high H2 storage capacity and CO2 capture, but the sensor ability for detecting individual gas molecules. The superior adsorption behaviors of graphene and graphene-based materials might be attributable to their large specific surface area and special interaction between electron-donor and acceptor molecules.

![Schematic representation of graphene: which is the fundamental starting material for a variety of fullerene materials, buckyballs, carbon nanotubes, and graphite [7].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f002.jpg)
![Scanning tunneling microscope topographs (0.8 V sample bias, 100 pA) of nominally 1 mL epitaxial graphene on SiC(0001). Top: image showing large flat regions of 6√3 × 6√3 reconstruction and regions where the reconstruction has not fully formed. Next-layer islands are also seen. Bottom: a region of 6√3 × 6√3 reconstruction, imaged through the overlying graphene layer (detailed information is presented in [19]).](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f003.jpg)
![(a) Schematic of the roll-based production of graphene films grown on a copper foil. The process includes adhesion of polymer supports, copper etching (rinsing) and dry transfer-printing on a target substrate. A wet-chemical doping can be carried out using a setup similar to that used for etching. (b) Roll-to-roll transfer of graphene films from a thermal release tape to a positron emission tomography (PET) film at 120℃. (c) A transparent ultra large-area graphene film transferred on a 35-in. PET sheet. (d) An assembled graphene/PET touch panel showing outstanding flexibility [27].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f004.jpg)

![Novoselov et al. [1] were the first to observe a single layer graphene; (a) photograph (in normal white light) of a relatively large multilayer graphene flake with a thickness of about 3 nm on top of an oxidized Si wafer, (b) atomic force microscope (AFM) image of 2 μm by 2 μm area of this flake near its edge (colors: dark brown, SiO2 surface; orange, 3 nm height above the SiO2 surface), (c) AFM image of single-layer graphene (colors: dark brown, SiO2 surface; brown-red (central area), 0.8 nm height; yellow-brown (bottom left), 1.2 nm; orange (top left), 2.5 nm. Notice the folded part of the film near the bottom, which exhibits a differential height of about 0.4 nm).](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f006.jpg)
![A proposed reaction pathway for epoxide reduction with hydrazine [49].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f007.jpg)
![Aberration-corrected transmission electron microscope image of a single sheet of suspended graphene oxide; (a) the oxidized region of the material, (b) the graphitic region, and (c) the atomic structure of graphite oxide’s region [57].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f008.jpg)
![Schematic representation of the mechanisms involved in “gasothermal” exfoliation techniques [60].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f009.jpg)
![Nitrogen/77 K adsorption-desorption isotherms of graphene oxide prepared from Srinivas et al. [87].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f012.jpg)
![Transmission electron microscope (a) and scanning electron microscope (b) images of graphene oxide prepared from Srinivas et al. [87].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f013.jpg)
![The multi-binding hydrogen adsorption model of Ti-anchored graphene oxide [94].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f015.jpg)
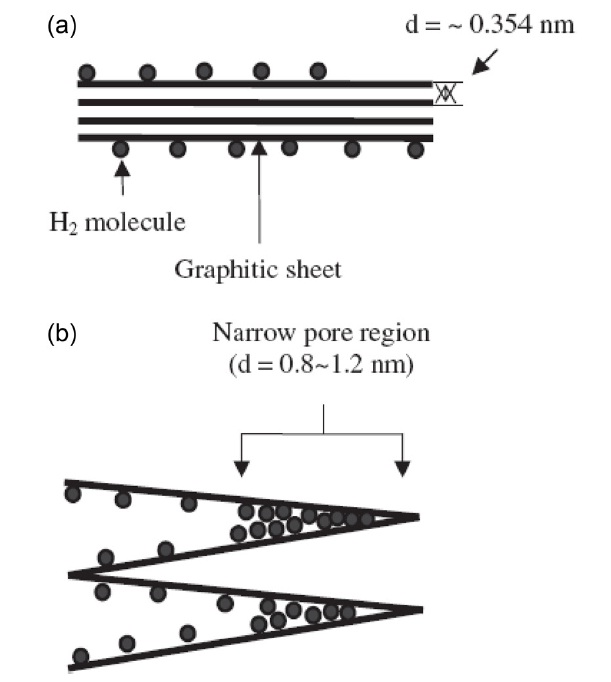
![A schematic of the hydrogen storage mechanism on metal/ carbon surfaces: (a) the accumulation of hydrogen molecules on metal/ carbon surfaces, (b) the dispersion of hydrogen molecules from metals to carbon surfaces by dipole-induced effects [95].](http://oak.go.kr/repository/journal/10993/HGTSB6_2012_v13n2_73_f018.jpg)