Nanotechnology is referred to as a science that makes a new system through which matter is manipulated on a nanoscale [1], and great advances have been made in the field of nanotechnology over the last decade. It is one of the most advancing technologies of the 21st century and is thought to occupy a leading position in industry. Engineered nanomaterials are already being used in various industrial fields such as sporting goods, cosmetics, and electronics and will be increasingly applied to medical fields such as diagnosis, cancer treatment, and drug delivery [2,3]. Regardless of these extensive applications, many concerns about the safety and the potential risks of engineered nanomaterials associated with human health have been raised [4].
Zinc oxid (ZnO) is one of the most exciting materials because of its versatile properties such as its semiconducting, biosafe, and biocompatible natures. ZnO is also considered to be a “GRAS” (generally recognized as safe) subst-ance by the Food and Drug Administration (FDA). Because of these properties, ZnO can be safely used in medical fields such as cancer therapy and drug delivery vehicles [5].
In this study, we examined whether ZnO makes a difference when it is combined with
2.1. Preparation of Hominis Placenta pharmacopuncture solutions (HPPS)
HPPS was prepared by using the following protocol provided by the Korean Pharmacopuncture Institute. Briefly,
2.2. Synthesis of ZnO nanoparticles (ZnO NP)
ZnO NPs were synthesized by using a low-temperature solution method with zinc acetate dihydrate (ZnAc), hexamethylenetetramine (HMTA), and LiOH [11]. For synthesizing fluorescein isothiocyanate (FITC)-conjugated ZnO NPs, a 3 ml FITC solution (3 mM) was added to a solution of 0.1 M ZnAc and 0.05 M HMTA. Subsequently, a 1.0 M LiOH solution was slowly added to the above solution to pH 10 and was refluxed at 90℃ for 3 hrs. The precipitates were washed and then airdried. The structure and the crystallinity of the as-synthesized ZnO NPs were determined by using transmission electron microscopy (TEM) and Xray diffraction (XRD) in the range of 20-70° at an 8°/min scanning speed.
The RAW 264.7 cells were obtained from ATCC (American Type Culture Collection, Rockville, MD, USA), were cultured in Dulbecco’s modi ed Eagle’s medium (DMEM) containing L-glutamine (200 mg/L) supplemented with 10 % (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and 100
2.4. Measurement of intracellular ROS generation
Cells, 1 x 106/ml, were cultured under the same conditions as in the flow cytometric analysis. ZnO NPs (1
2.5. Reporter construct and luciferase assay
To measure the NF-
2.6. Isolation of total RNA from cells and RT-PCR
We isolated total RNA by using Trizol reagent through the manufacturer’s instructions (Invitrogen, Carlslab, CA, USA). After the concentration of RNA had been determined by using a spectrophotometer, we made cDNA by using reverse-transcription from 1
2.7. Cellular uptake and FACS analysis
RAW 264.7 cells (1 × 106 cells/ml) were plated in a 96-well plate and were incubated in 5% CO2 at 37℃. After a 24 hrs incubation, the culture medium was replaced with 100
2.8. Transmission electron microscopy (TEM)
Cells were cultured at a density of 1 × 106 cells/ml in a culture medium, each medium containing samples at a concentration of 100
All data are expressed as means ± standard deviations of at least three independent experiments. For comparison among groups, a one-way analysis of variance (ANOVA) test was used (with the assistance of Graph Pad Software, Inc., San Diego, CA).
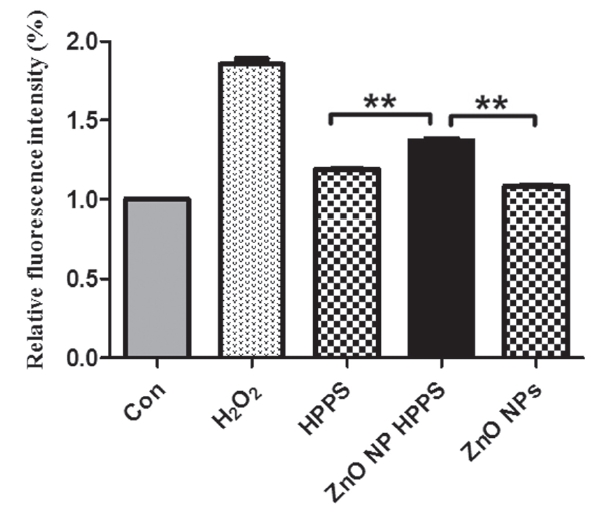
3.1. ZnO NP HPPS significantly induced intracellular ROS production
To measure the effects of ZnO NP HPPS on the intracellular ROS production, we treated RAW 264.7 cells with 1
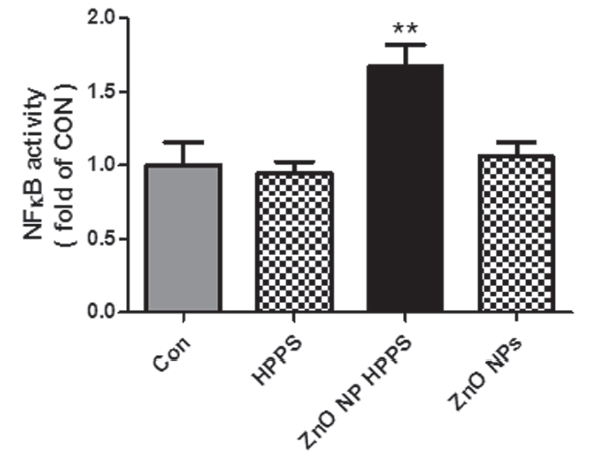
3.2. ZnO NP HPPS significantly increased the NF-κB activation
To evaluate the potential of ZnO NP HPPS to induce cellular activation, we measured the activation of NF-
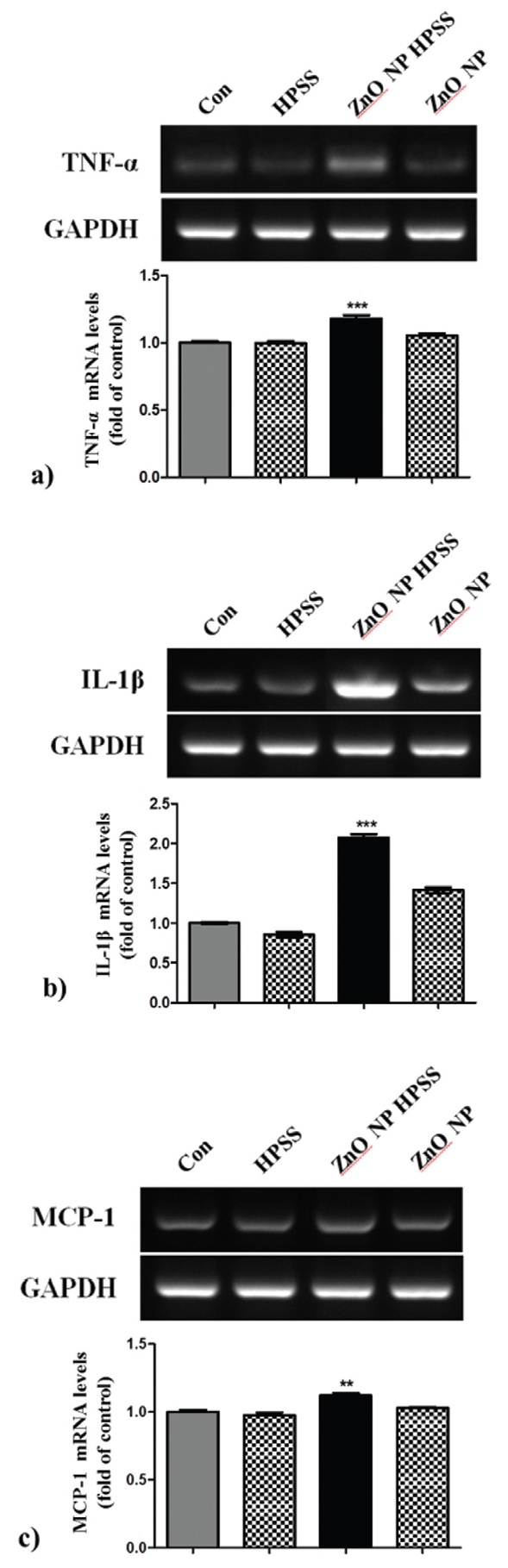
3.3. ZnO NP HPPS increased the expression of NF-κB dependent cytokine mRNA
To evaluate whether ZnO NP HPPS could modulate TNF-
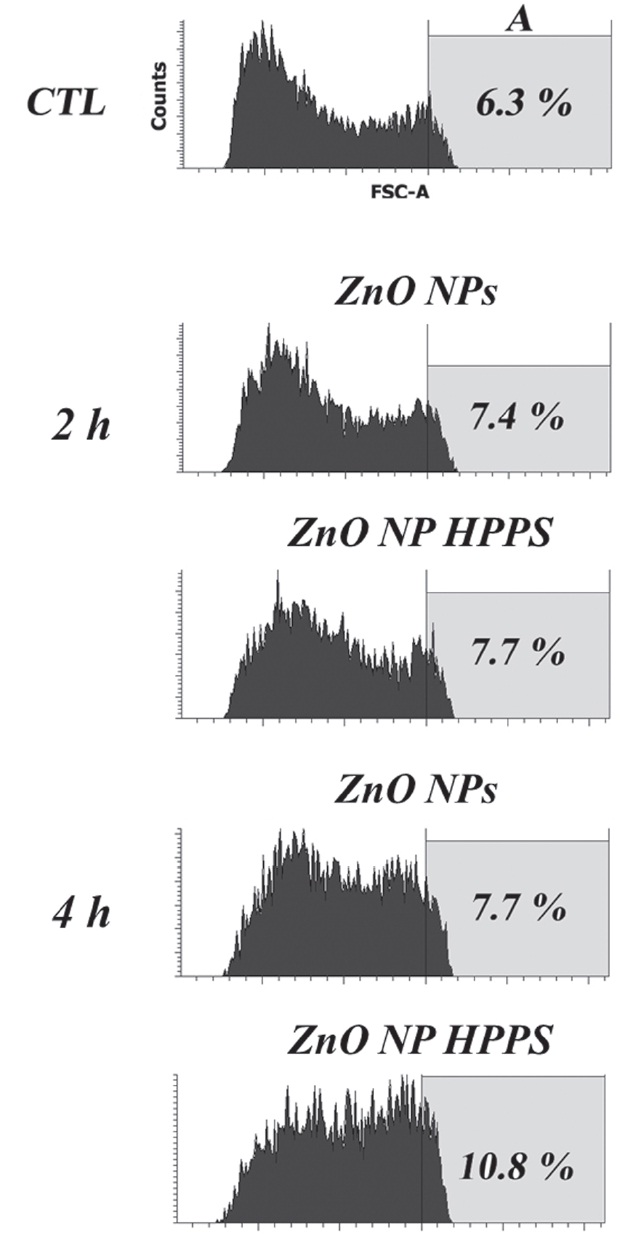
3.4. Uptake of ZnO NP HPPS and ZnO NPs (by FACS analysis)
To examine the cellular uptake and the permeation of the ZnO NP HPPS through the cell membrane, we treated the RAW 264.7 cells with 1
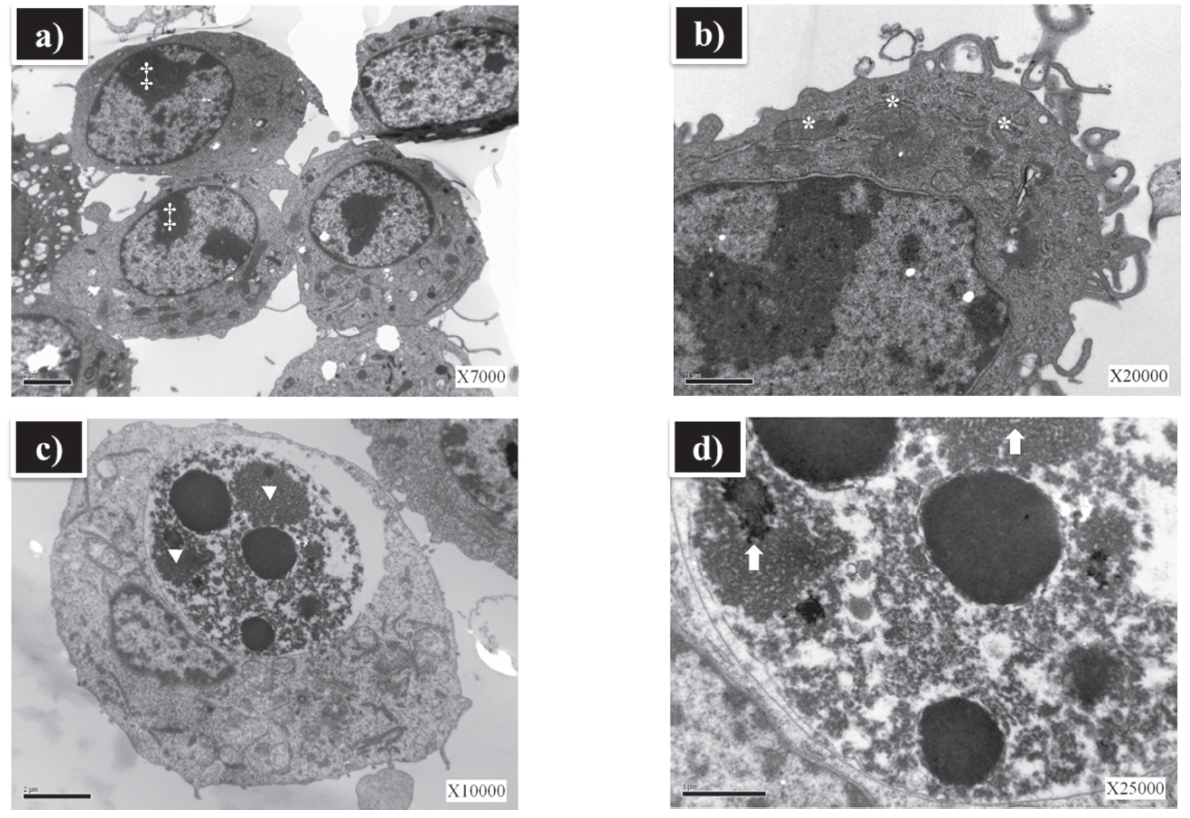
3.5. ZnO NP HPPS caused structural alterations in RAW 264.7 cells
For the analysis of subcellular morphologic changes, ZnO NP and ZnO NP HPPS treated cells were examined by transmission electron microscopy (TEM). RAW 264.7 cells were treated with either ZnO NP or ZnO NP HPPS for 4 hrs. There were no ultrastructural alterations in the cells treated with ZnO NP. The integrity of subcellular structures such as plasma and nuclear membrane were normal (Fig. 5a, b). However, significant alterations were observed in the cells treated with ZnO NP HPPS. Variations in size and shape of the cells were prominent, and large inclusions were observed in the lysosome (Fig. 5c, d).
Nanotechnology is an engineering system that manipulates matter at a molecular scale. Engineered nanomaterials have already been used in industry such as sports, cosmetics, and electronics. In the last decade, great advances have been made in nanomedicinal field. Recent studies have focused on using nanoparticles for imaging, tissue engineering, and drug delivery [12-15].
Delivering therapeutic agents into cells can be highly inefficient because of diverse physicochemical properties of therapeutic agents. Recently there were many extensive investigations in the field of drug delivery systems for the purpose of improving and facilitating drug efficacy. Nanomaterials can modify the physicochemical properties of a drug so to provide an opportunity for increasing uptake and interaction with target cells. Therefore, it is important to ensure that these agents do not cause any adverse effect. However, there are growing concerns about the toxic effects of engineered nanomaterials on human health because of their unusual physicochemical properties. There are some consensuses that deal with potential risks associated with inhalation of nanoparticles [16,17].
Nanoscale ZnO used in many applications in daily life is believed to be nontoxic and biocompatible. Recently, attentions have focused on the Zinc Oxide nanoparticles (ZnO NPs) because of its own unique biosafe and biocompatible properties [5].
HPPS is one of the most widely used herb-acupunctural material to replenish vital essence and blood in Korean traditional medicine [18]. Several studies about therapeutic effectiveness of HPPS were reported. HPPS has a protective effect against osteoporosis [8], growth-promoting activity on the nerve regeneration [9], protective effect on radiation enteropathy [19], and capacity to regulate bone resorption [20].
In this study, we examined whether ZnO NO HPPS, a combining form of ZnO NP and HPPS, has any special effects over HPPS. Oxidative stress was regarded as a major cytotoxic standard caused by nanomaterials [2]. Previous studies showed that ZnO NP induces ROS production [21-23]. However, our results revealed that ZnO NP did not increase intracellular ROS production, but ZnO NP HPPS up-regulated the intracellular ROS level (Fig. 1). It is well known that NF-
In conclusion, our results show that each of the HPPS or ZnO NP did not induce any remarkable changes on RAW 264.7 cells, while ZnO NP HPPS, a combined form of ZnO NP and HPPS, significantly activated the RAW 264.7 cells. ZnO NP HPPS increased the intracellular ROS production, NF-
ROS production by phagocytes normally occurs in response to a variety of infectious and proinflammatory stimuli. This process forms essential part of the innate immune system [27]. From the result that ZnO NP HPPS has effectively stimulated RAW 264.7 cells through ROS production and NF-