Recently, lead zirconium titanate (PZT) films have been used in various applications, such as pyroelectric infrared detectors,piezoelectric actuators, micro-electro-mechanical system and nonvolatile ferroelectric random access memories, due to their high piezoelectric constants [1-4]. The composition of the PZT films is determined to be due to their great piezoelectric characteristics around the rhombohedral-tetragonal morphotropic phase boundary (about Zr:Ti = 52:48) [5,6]. The PZT films have been prepared by sol-gel, screen printing, pulsed laser deposition, radio frequency sputtering and metal-organic decomposition,etc. [7-9]. Among the preparation methods, the sol-gel method has many advantages such as high productivity, high purity, good homogeneity, control of chemical composition and microstructure, low-temperature processing, and low cost. The sol-gel technique, which consists of film deposition by dip or spin coating and pyrolysis by heat treatment, has been broadly used for this purpose [10].
The PZT films often require submicron thickness or over for industrial applications such as actuators, harvesters, sensors, etc.The sol-gel derived thick film can be obtained through repetitive sol coating and several heat treatments by using a low viscosity solution. However, the fabrications of the repetitively coated films are not compatible with industry due to their complex processes.
Moreover, crack formation tends to occur during heat treatment in thick gel films. The limitation on film thickness for avoiding crack formation is one of the factors restricting the ap-
[Table 1.] The PZT solutions of PVP molar ratios.

The PZT solutions of PVP molar ratios.
plication of the sol- gel technique to the industrial production of ceramic coatings [11,12].
Most of the as-deposited gel films are built up with metalloxane polymers and are porous. A condensation reaction, which results from collapse and film densification, occurs when gel films are treated by heating. Thus, the reason for crack formation is the evolution of in-plane tensile stress due to the thermal expansion coefficient and shrinkage of pores.
The viscosity of the solution cannot be increased by reducing the solvent contents because of its low starting solubility. Therefore, conventional sol-gel coating can normally only achieve crack-free films less than 0.1 μm in thickness with a single-step process [13,14].
Saegusa and Chujo [15,16] reported that organic polymers with amide groups, such as poly(N-vinylpyrrolidone) (PVP), poly(N-acetylethylenimine), poly(2-methyl-2-oxazoline), and poly(N,N-dimethylacrylamide), can be hybridized with metalloxane polymers on a molecular scale. It can be bonded with metalloxane polymers through strong hydrogen bonding. Thus, the addition of organic polymers with amide groups, such as PVP in alkoxide solutions can suppress the condensation reaction in gel films during heat treatment, increase the viscosity of alkoxide solutions, and reduce the in-plane stress in films, and it can allow crack-free films over submicron thickness.
In this study, PZT films of various thicknesses have been prepared from stable PZT solutions containing various molar ratios of PVP. This has been followed by third-step heat treatment with drying at 150℃ and 350℃, and annealing at 650℃. PZT films that are thick (1 μm) and dense have been realized on the Pt/Ti/SiO2/Si substrate using repetitive coating. The effects of the contents of PVP on the formation of the PZT films have been investigated.
PVP of 1.3 × 106 in viscosity-average molecular weight was used. The molar ratio for PVP was defined for the monomer. The starting materials were lead acetate trihydrate (Pb(CH3COO)2·3H2O), zirconium acetylacetonate (Zr(OC3H7 n)4), and titanium diisopropoxide bis(acetylacetonate) 75wt%in isopropanol (Ti(OC3H7
in CH3(CH2)2OH under stirring. Each process was maintained at 80℃ for 30 minutes during stirring. The precursors were mixed, and then the mixture solution was continuously stirred under the same condition for 2 hours. Finally, aging was carried out at room temperature for 24 hours. Throughout the process, the solution was transparent, and showed a highly stable state.
Table 1 summarizes the PZT sols prepared from the solutions with PVP molar ratios of 0, 0.25, 0.5, 0.75 and 1.
The PZT films, which are based on the Pb1.1(Zr0.52,Ti0.48)O3 composition, were prepared on a Pt/Ti/SiO2/Si substrate by repetitive spin-coating. The PZT sol was spin-coated at a rate of 4,000 rpm for 40 seconds and the films were pyrolized by a two-step heat treatment at 150℃ and 350℃ for 10 minutes per-process on a hot plate in order to remove solvents and organics.
Finally, the gel films were annealed at 600℃ for 10 minutes by rapid thermal annealing.
For the measurements, platinum electrodes 0.2 mm in diameter were deposited on the film surface by sputtering through a shadow mask.
2.2 Measurement and characterization
To determine the pyrolysis and crystallization temperature, thermalgravimetric analysis (TGA) and differential scanning calorimetry (DSC) was performed on a solution with a heating rate of 10℃/min in air using a thermal analyzer. The surface and cross-sectional morphologies of the films were measured using the environmental scanning electron microscope. The crystal structures of the films were evaluated by X-ray diffraction (XRD) measurement. The dielectric constants of the films were measured at 100 kHz to 1 MHz using a precision impedance analyzer (4294A). The polarization-electric field characteristics were evaluated using a RT66A.
3.1 Effect of adding PVP content
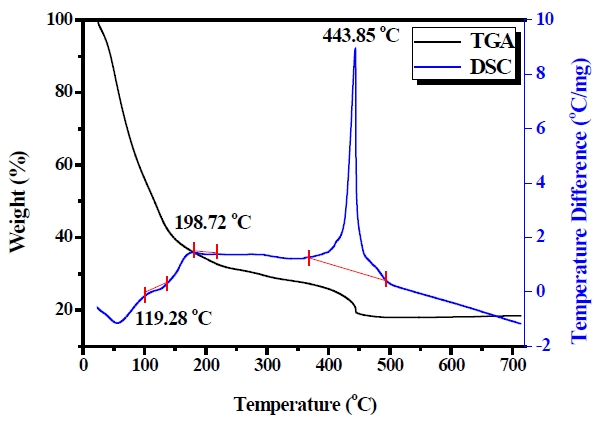
Pyrolysis conditions were based on TGA-DSC analysis for the sols. Figure 1 shows the TGA-DTA curves of the PZT 2 sol from 10 to 800℃. The TGA-DSC analysis of the sols shows that most of the organics and other volatiles are removed at temperatures <550℃. The strong endotherm at < 200℃ and the corresponding weight loss is due to evaporation of polymer reactions within the gel. Complex reactions of organics occur within the temperature range of 200-550℃. An exothermic peak around 200℃ is attribut-
able to the burning of organic moieties, and another exothermic peak around 450℃ shows the crystallization of the PZT phase. Figure 1 does not show the characteristics of the other samples, because the characteristics of the other PZT sols indicated similar data to the PZT 2 sol.
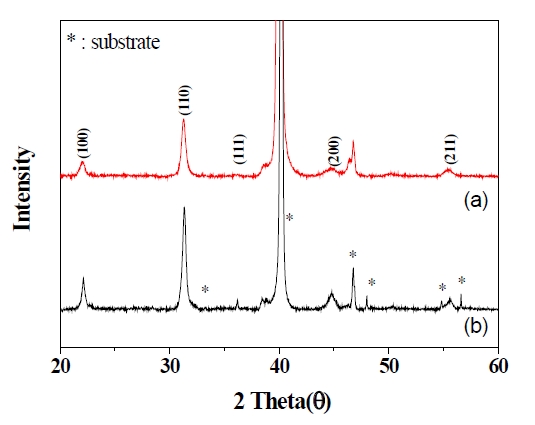
Figure 2 shows the XRD patterns of the PZT films prepared from the solution with PVP (Fig.2 (a)) and without PVP (Fig.2(b)). All patterns show the single-phase PZT, although the PZT solution included the PVP composition.
Also, Fig. 2 does not show the characteristics of the other samples, because the characteristics of PZT films with PVP content indicated similar data to the PZT 2 film.
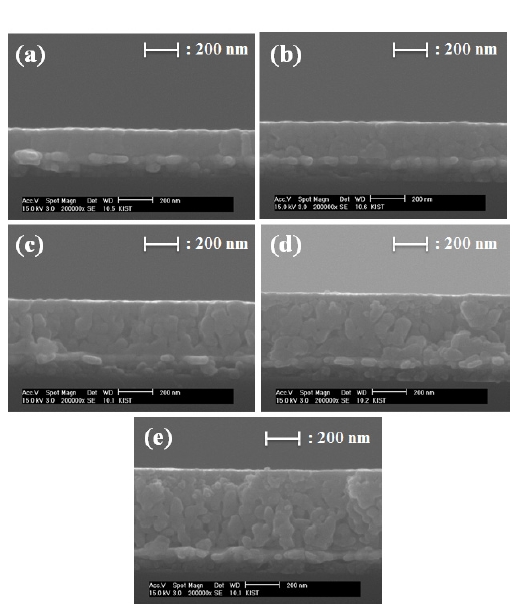
The cross-sectional images of single-step coated PZT films deposited on the Pt/Ti/SiO2/Si substrate with various PVP molar ratios are shown in Fig. 3The thicknesses of PZT films increase with the increase of PVP content, because of the increased viscosity of sols. However, the PZT 4 and 5 films suffer from crack formation when prepared by a single-step process, because PZT films with a PVP molar ratio of over 0.5 contain bigger pores during heat treatment.
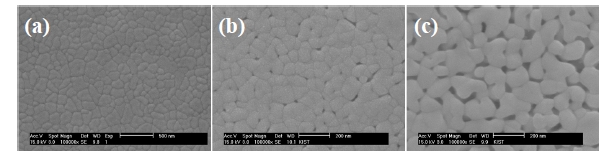
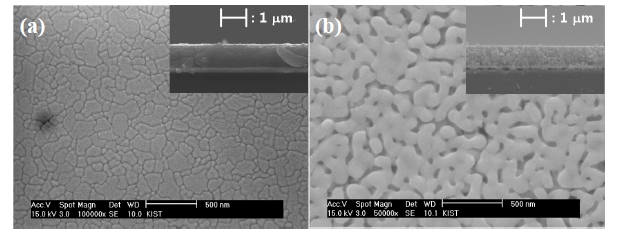
Figure 4 shows the surface SEM images of PZT films with 3 coating layers and various PVP molar ratios deposited on Pt/Ti/
SiO2/Si. The PZT 4 and 5 films are not shown in this figure due to their crack-formation. The PZT 1 film shows large grains, which were over 100 nm. Nanosized pores were observed in the films with PVP, which are expected to have aided in relaxation of stress during annealing. The porosities of the PZT films increased with increasing PVP molar ratios in Figs. 4(b) and (c).
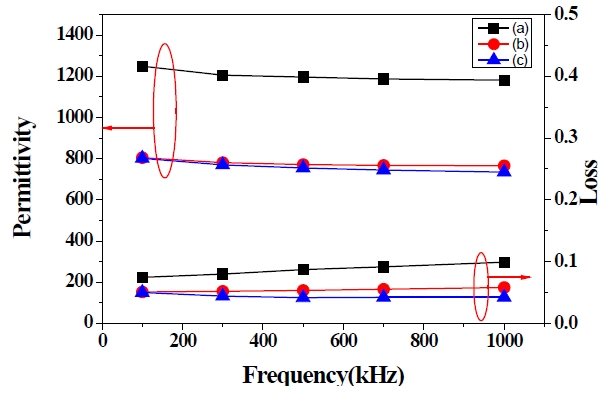
Figure 5 shows PZT films with 3 coating layers and various PVP molar ratios annealed at 650℃. The thicknesses of the PZT 1, 2 and 3 films were 295 nm, 590 nm and 996 nm, respectively. However, the dielectric properties of the PZT 2 and 3 films are nearly unchanged, as shown in Fig. 5, even though the PVP molar ratios increased between the PZT 2 and 3 films.
3.2 Characteristics of 1 μm PZT films
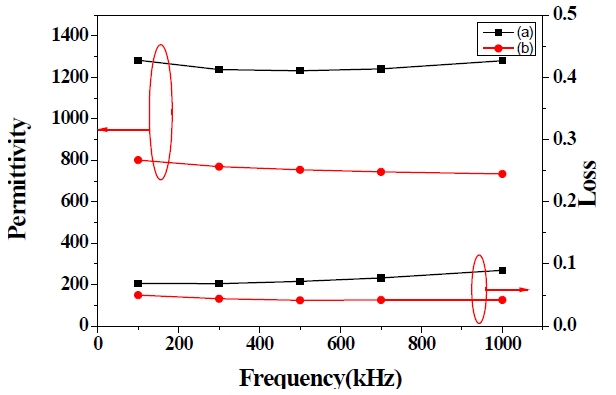
The 1 μm thicknesses of the PZT films were prepared by using repetitive coating. The surface and cross-sectional microstructures of the PZT films are shown in Fig. 6 The thicknesses of the PZT 1 and 3 films are 970 nm and 990 nm, respectively. Figure 6(a) inset shows a cross-sectional image of the PZT 1 film, which was prepared with 8 coating layers for a film of 1 μm thickness. However, the PZT 3 film was prepared with only 3 coating layers for a film of 1 μm thickness, as shown in Fig.6 (b) inset.
The dielectric constant and loss of the 1 μm PZT 1 and 3 films are shown in Fig. 7 The permittivity of the 1 μm PZT films with PVP molar ratios of 0 and 0.5 are 1,280 and 734 at 1 MHz, respectively. The dielectric loss of the PZT films with PVP molar ratios of 0 and 0.5 are 0.089 and 0.042 at 1 MHz, respectively. The permittivity remained nearly unchanged throughout the measured frequency up to 1 MHz. However, the permittivity of the PZT film without PVP shows greater improvement than the PZT film with the PVP molar ratio of 0.5. It can be considered that the pores remained inside the PZT film with PVP, and the pores affect the electrical properties of PZT films.
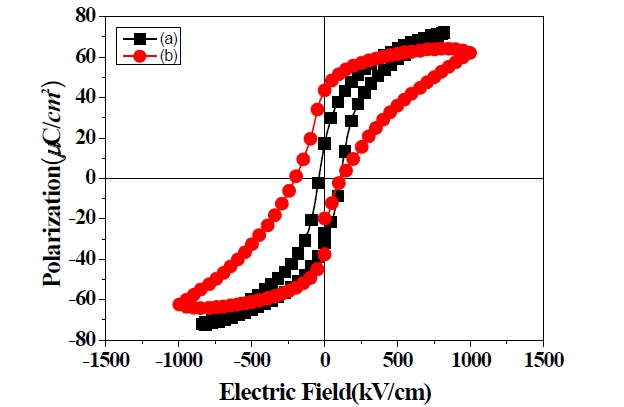
The ferroelectric hysteresis loops of the PZT 1 and 3 films are shown in Fig. 8 The average remnant polarization of the PZT 3
film is 40.5 μC/cm2, and the average coercive field is 156 kV/cm under an applied electric field of 1,000 kV/cm. The average remnant polarization of the PZT 1 film is 26 μC/cm2, and the average coercive field is 72 kV/cm under an applied electric field of 750 kV/cm. The swollen appearance of the hysteresis loop due to the overhigh Pr and overlarge Ec can be attributed to the large electric leakage of the PZT film with PVP content because of the pores.
The 1 μm thickness lead zirconate titanate (Pb(Zr0.52Ti0.48)O3;PZT 52/48) films containing a PVP molar ratio of 0.5 with the perovskite structure, were successfully prepared by coating 3 times at 650℃. The PZT film with PVP content shows a swollen hysteresis loop due to the large electric leakage content, because of its porosities. The dielectric constant and loss of the PZT film with PVP content are 734 and 0.042 at 1 MHz. The optimized Pr and Ec of the PZT film with PVP content are 40.5 μC/cm2 and 156 kV/cm under an applied electric field of 1,000 kV/cm.