The global population of elderly people has been growing rapidly over the past few decades. In Europe, one third of the population will be over 65 years old by the year 2035 [1].China, on the other hand, is one of the countries faced with the fastest growing aging problem. Over one-fifth of the world's elderly population (aged 65 and over) lives in China and this proportion is envisaged to increase up to 24% by 2020. Furthermore,the problem is aggravated by the fact that the number of elderly people leading solitary lives is also increasing and that a large percentage of them are suffering from either chronic diseases or varying degrees of disability.
Chronic diseases have been reckoned to be the world’s leading cause of death and disability, and will account for almost three-fourths of all deaths by 2020. Each year, the number of deaths caused by cardiovascular diseases and hypertension is estimated to be 16.7 million and 7.1 million respectively. The population of diabetic adults is expected to reach 300 million by 2025 [2]. The cumulative effects of all these factors will be serious social problems, which in turn will place a severe financial burden on healthcare services. As a result, there is an increasing demand for a shift from the conventional hospital-centered healthcare system for treating symptoms to a new healthcare system that emphasizes early detection of risk factors, early diagnosis, and early treatment [3].
In order to collect health information before symptoms of illnesses appear, new acquisition systems are needed. In addition to systems for acquiring high-resolution images and genetic information, Wearable Intelligent Systems for e-Health (WISEs)have been proposed because of their capability for exercising long-term, continuous, and unobtrusive collection of health information. Since some of the physiological parameters (e.g.blood pressure and blood glucose) vary greatly according to the daily activities that the subjects are involved in, the development of novel WISEs that can effectively monitor the vital signs continuously over a long period is in some cases crucial to understanding the progression of some chronic symptoms in the elderly. WISEs can also capture transient features and early indicators of health deterioration.
The objectives of this paper are 1) to review recent development of WISEs, including breakthrough technologies and technical challenges that remain to be solved and 2) to report the results of a novel application of the wearable technologies in capturing transient cardiovascular features during water drinking. The rest of paper is organized as follows: Section III reviews the state-ofthe-art of WISEs. Section IV discusses the development of textile sensors, which can be considered as a major breakthrough technology in WISEs. Section V focuses on the problems that remain to be solved for WISEs, i.e., the issue of motion artifacts and potential techniques for reducing them. Section VI reports the latest results of our recent study on a novel application of wearable technologies, which is to measure the transient variations of multiple cardiovascular parameters (i.e., heart rate and pulse transit time) during water drinking. Finally, the conclusions and other potentially viable applications of wearable medical devices are presented in Section VII.
WISEs consist of various kinds of sensors such as microelectronic,biochemical, or textile-based sensors embedded or integrated into wearable objects (such as gloves, finger rings,wristband, ear-worn devices, waist-belt, or cloth, etc.) for the noninvasive monitoring of physiological signals and parameters,such as electrocardiogram (ECG), biopotentials, photoplethysmogram(PPG), heart rate (HR), blood pressure (BP), blood glucose(BG), and blood oxygen saturation (SpO2), respiration and body activity. Recently, a wearable system incorporating an ultrasound sensor [4] has also been developed for the cardiopulmonary activity monitoring in emergency.
Health information acquired by WISEs can be used for realtime,on-site detection of physiological conditions of the elderly and can also be transferred wirelessly to remote medical centers for early diagnosis. By the same token, remote medical centers can, in turn send early treatment advice to the patients in realtime.
WISEs should entail five characteristics, namely MINDS [5]:1) Miniaturized: to include innovative approaches and measurement principles for acquiring physiological information as well as to incorporate small-sized and low-power integrated circuit designs;2) Intelligent: to be able to learn and timely evaluate an individual’s health conditions, and issue alerts whenever appropriate; 3)Networked: to connect on-body sensors by a body area network(BAN) and to wirelessly hook up with remote medical centers;4) Digitalized: to incorporate new bio-sampling and bio-signal processing theories to handle the massive multi-modal health information; and 5) Standardized: to allow standard communication and evaluation protocols for ensuring interoperability, measurement accuracy and information integrity.
A number of WISEs have been developed by various research groups, including a smart garment for measuring HR,breathing rate (BR), body temperature (BT), and SpO2 [6] an earlobe PPG detector [7], a wrist-worn device AMON for BP, ECG, SpO2 monitoring [8], a glove for monitoring skin temperature (SKT), PPG, and galvanic skin response (GSR) [9],a respiration waist belt [10] and a ring-type PPG sensor [11].
The main research focus of our team has been on the development of a cuff-less BP estimation technique based on the measurement of pulse transit time (PTT). The technology has been implemented as a cuff-less BP watch previously by one of our industrial sponsors [12] and as an h-shirt developed
by our research center for monitoring PPG, ECG, and BP [13].
Fig. 1 shows some of the device prototypes that can be used in WISEs. In order to develop these prototypes, new sensors and innovative measurement principles as opposed to the conventional medical devices used in the hospitals are often incorporated, as summarized below.
Non-contact wireless ECG sensors based on the principle of capacitive coupling are now becoming washable and fully integrated with clothing and wearable accessories [14-16]. These wireless sensors overcome the shortcomings of traditional wet adhesive electrodes and can operate without directly contacting the skin surface. The sensors can be manufactured in the form of fabric by weaving or knitting conductive yarn/rubber/ink electrodes [17].
In addition to arrhythmia, HR and heart rate variability(HRV) are also indicators of health [18-20]. HR and HRV data can be extracted from ECG, PPG, remotely by microwave radar sensors based on the doppler effect [21] and most recently by applying independent component analysis (ICA), a blind source separation method, on video images of people’s faces [22].
Respiration is most commonly measured by sensors integrated into a belt or garment. The types of sensors used include impedance pneumographic, inductive plethysmographic, piezoresistive,piezoelectric and textile-based capacitive sensors [23-25].Respiration rate can also be extracted from other physiological signals such as ECG and PPG [26].
The most popular method for non-invasive estimation of SpO2 is by means of photoplethysmography. The method is based on the difference in absorption of two wavelengths of light by the pulsatile arteriolar blood flow. Sensors have been integrated into finger rings, earlobe devices, foreheads, wristworn devices and shirts in wearable application [11, 16, 17]. A wearable imaging device is also able to detect SpO2 and blood volume non-invasively by functional near-infrared (fNIR)spectroscopy [27].
Diabetes is a common disease in the elderly population. In particular, sufferers of type I diabetes require daily BG measurements followed by insulin injections. These patients’ quality of life can be greatly improved by using feedback system with a small insulin pump to regulate the insulin delivery based on the measured BG levels. The system requires the patient’s glycemia to be measured accurately and continuously such that the insulin infusion rate and dosage can be adjusted accordingly.In recent years, several approaches for continuous monitoring have been developed, such as the subcutaneous needle sensor.Fig. 2 shows a needle-type glucose sensor used for a wearable artificial endocrine pancreas [28]. This sensor is placed in the
subcutaneous tissue and measures subcutaneous glucose concentration continuously. Another study reported a wearable glucose monitor based on SC open-flow microperfusion techniques,including handling of liquids, glucose sensors and electronics for motor control, sensor read-out, and communication [29].Nevertheless, the above methods are invasive.
Advanced technologies for BG monitoring focus on needlefree,transcutaneous measurements [30]. A number of methods have been demonstrated to have great potential for the noninvasive and continuous monitoring of BG, e.g., by reverse iontophoresis, impedance spectroscopy, photoacoustic spectroscopy,near infrared spectroscopy, electrophoresis, enzyme-based direct electron transfer [30, 31], some of which have been implemented in “watch-like” wrist-worn devices.
>
E. Other Biochemical Measurements
In addition to BG, biochemical measurements of other body fluids, such as blood, sweat and urine, are also under active development. Real-time monitoring of the pH of sweat is usually performed using wearable micro-fluidic devices [32,33]. The microchip was fabricated using polymer and can also be manufactured in textile form [32].
Hypertension is another common disease found in the elderly population. Elevated BP increases the workload of the heart and scars the artery walls. Increases in either BP or BP variability(BPV) are partly responsible for various cardiovascular events[34]. Nevertheless, most individuals with hypertension experience no symptoms, which often make them overlook their ailment.Thus, early detection of BP for health condition assessment by wearable devices before a severe event occurs is very important.
Technologies advanced in wearable BP monitoring focuses on continuous and noninvasive measurement without using a cuff. Cuff-less BP can be measured from the radial pulse waveform by arterial tonometry [35]. Another promising technique for cuff-less BP is based on the estimation of PTT[12, 36]. Such technologies can be integrated with a personal
digital assistant (PDA), a watch and a finger ring for cuffless monitoring of arterial BP [12, 35, 36]. Together with electronic textile (e-textile)-based technologies, the technique can also be used to design a shirt for long-term, hands-free continuous monitoring of BP, as shown in Fig. 3 [13].
III. E-TEXTILE SENSORS FOR WISES
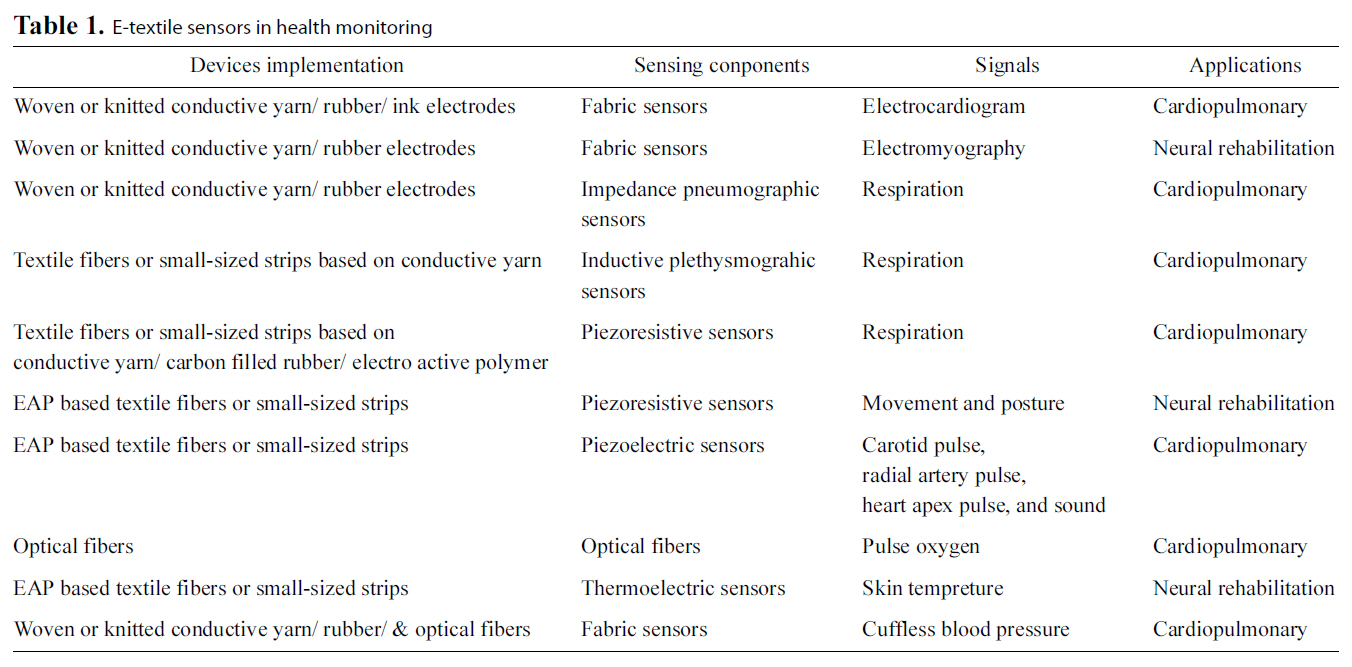
A breakthrough technique adopted in many WISEs is the electronic textile (e-textile) sensing technology, which has led to a number of innovative methods for measuring various physiological signals and parameters, as reviewed in Section II.Conductive and functional materials in the form of fiber and yarn can now be integrated into garments and used as sensors and connectors [14]. Smart shirts based on utilization of etextiles having the same appearance as common clothes are
[Table 1.] E-textile sensors in health monitoring
E-textile sensors in health monitoring
capable of vital signs monitoring without causing deterioration of fabric behavior [37]. Table 1 summarizes typical usage of etextile sensors in health monitoring.
Conductive yarn, conductive rubber, and conductive ink have been developed into sensors [23] or used as an interconnection substrate [37] in WISEs. Conductive yarns and fibers are made by mixing pure metallic or natural fibers with conductive materials [24]. Pure metallic yarns can be made of composite stainless steel or fine continuous conductive metal-alloy [17].Combination of fibers with conductive materials can be completed by the following methods [37]: 1) Fibers filled with conductive material (e.g., carbon or metallic particles); 2)Fibers coated with conductive polymers or metal; and 3) Fibers spun with thin metallic or plastic conductive threads. Currently,most textile-based sensors (fabric sensors) for health monitoring are achieved by knitting or embroidering conductive yarns [23].Conductive rubber is made by filling a silicon rubber with conductive materials such as carbon or metals. They are usually called carbon or metal loaded rubber (CLR/MLR) [23, 38], and can be integrated into textile substrates by coating technologies.Researchers at North Carolina State University proposed another method of making textile electrodes for WISEs, which is by silk-screen printing conductive inks (Ag or AgCl) onto non-woven substrates [17].
The conductive properties are exploited in order to capture physiological electric activity (such as ECG, electroencephalogram and electromyogram) from the surface of the skin [23, 39-41].Impedance pneumography for monitoring respiration is accomplished by locating two e-textile sensors on the chest, and inputting a low-current high-frequency sinusoidal signal between the sensors. Impedance across the chest was evaluated by measuring the voltage drop during breathing [23, 42]. This is the long-established standard method for respiration monitoring
[23]. Another new method for respiration monitoring is based on inductive plethysmography. The respiration pattern is assessed by measuring the change in inductance with an inductive sensor,which is usually designed in the form of a belt, constituting a wire formed in a sinusoidal pattern [23, 43]. Continuous cuffless BP monitoring based on bio-models has been studied based on measurement of PTT from ECG obtained by textile electrodes and PPG by a photoreflective sensor [13].
Piezoresistive, piezoelectric, chemoresistive, thermoelectric,photoelectric materials and other functional materials in the form of textile fibers can be used for sensors, actuators electronic components, and power sources based on its physical effect [24]. The changes of the resistance of piezoresistive sensors when stretched and strained were used to evaluate biomechanical variables (such as respiration, movement, gesture and posture) [23, 44]. Carbon filled with rubber was successfully used for making a piezoresistive respiration sensor [23]. It is usually designed in the form of elastic strip.
The piezoelectric effect refers to the generation of current when stress is applied. Several kinds of accelerometers based on piezoelectric, piezoresistive materials have been widely used for physical activity monitoring, movement and fall detection[44, 45]. Piezoelectric sensors made of electroactive polymer(EAP) based textile fiber, can be used for monitoring carotid pulse, radial artery pulse, heart apex pulse, and sound detection[24]. These can also be applied as wearable power sources,textile keyboards, and general-purpose contact sensors (e.g., fall detectors) [24].
>
C. Integration of Textile-based Sensors with WISEs
Integration of textile-based sensors into clothing for health monitoring dates back to 1996 [46], when the Georgia Tech Wearable Motherboards was one of the first health applications integrated in a garment. The motherboard created a system for a soldier that is capable of providing unobtrusive monitoring of biological signals through textile sensors.
Another example of wearable health monitoring is the LifeShirt,which is a multi-function ambulatory system monitoring key physiological measurements, such as ECG, ventilation, respiratory rate, heart rate and activity, during daily living [29]. Other famous projects in this field include the MIThril LiveNet System [47], which is aimed at developing new techniques of human-computer interaction for body-worn applications. Since 2002, there have been a cluster of European Commission cofinanced projects on Smart Fabrics, Interactive Textiles (SFITs),as shown in Fig. 4, starting with “physiological parameteroriented projects” (e.g., WEALTHY, MyHeart, MERMOTH,and OFSETH) relying on textile-embedded sensors, and extending to biosensing textiles (BIOTEXs) [48], and a combination of signals (e.g., PROETEX) [49]. Currently, only a few wearable e-textile health monitoring systems (e.g., the VivoMetrics LifeShirt, Sensatex SmartShirt system, and MagIC) have been commercialized [15], while several more are still under development [23].
A technical challenge unique to WISEs is the accurate measurement during user movement. The signals acquired by wearable sensors are often corrupted by a patient’s motion. This motion artifact (MA) results in inaccurate estimation of required parameters, even when patient movements are very small, such as shivering. Generally, artifacts arising from skin stretch and the electrodes or sensors are the major causes of motion artifact [44]. At present, various methods have been studied for removing artifacts.
The most well-known method of motion artifact reduction is adaptive filtering [50-53]. Additional motion tracking sensor and signal processing procedures are needed for reducing motion artifacts. The signal acquired by this motion sensor becomes the reference input for the adaptive filter. The MIT group utilized a MEMS accelerometer as a reference channel to reconstruct the signals obtained from their finger-ring PPG sensor[50]. Another study compares the use of the force-sensitiveresistor sensor and the accelerometer as a reference of motion and found that the former better characterizes the probe-coupling artifacts between the skin and a wrist-type device [52].
The adaptive algorithms commonly used include the least mean square (LMS) and the recursive least squares adaptive methods. A study [45] compares the performance of these two algorithms and found that both algorithms produced similar improvements. Nevertheless, implementing the adaptive LMS algorithm is advantageous, since it requires significantly fewer operations. The advantages of adaptive filter methods include faster response time and better real-time performance, but additional sensors are required to detect the noise reference signal.More recently, a new method was reported that uses a synthetic noise reference signal generated from an MA corrupted PPG signal without any extra hardware such as accelerometers or additional source-detector pairs for noise reference signal generation[54].
Another common method is to use the signal processing algorithms directly, such as by wavelet transform and ICA. In past decades, the wavelet transform has been proven to be a useful tool for signal analysis [55]. The wavelet transform can be used as a decomposition of a signal in the time-frequency and time-scale planes. The general denoising procedure involves three steps: decomposition, thresholding, and reconstruction[56]. A comparative analysis [57] used different wavelets for the case of MA reduction from corrupted PPG signals, including Daubechies, biorthogonal, reverse biorthogonal, symlet,Coiflet types of wavelets. Experimental results revealed that the Daubechies wavelet resulted in excellent reduction of MA compared to other wavelets. However, the study in [58] revealed that wavelet transformation has limited applicability in restoring corrupted PPG signals for both HR and PTT measurements.
Recently, increased emphasis has been placed on ICA to reduce motion artifacts, because it does not require prior knowledge of the system [59, 60]. This method assumes that all source signal component pairs are mutually independent. A recent work, however, indicates that motion significantly affects arterial flow and therefore, care must be taken when applying ICA to light-based sensor data acquired from wearable platforms [61].
Some other techniques have also been found to be effective for reducing motion artifacts, including the smoothed pseudo Wigner-Ville distribution [62], the cycle-by-cycle Fourier series[63], the empirical mode decomposition [64] and the singular value decomposition method [65].
The minimum correlation discrete saturation transform(MCDST) algorithm based on an optical model derived from photon diffusion analysis was considered to be more robust under low Signal-to-Noise Ratios than the clinically verified motion-resistant algorithm discrete saturation transform (DST).Further, the experiment with varying motion severity demonstrates that MCDST has a slightly better performance than the DST algorithm [66]. Another experiment compared the LMS adaptive filter and the exponentially weighted least square(EWLS) adaptive filter with the MCDST. The experimental results indicate that both adaptive filters can perform better than the MCDST, and the EWLS adaptive filter performs better than the LMS adaptive filter in motion noise reduction [67].
Although there have been many attempts, the reduction of motion artifacts is still a major limitation in most practical implementations of WISEs. Further studies of the generation mechanisms of physiological signals and motion artifacts as well as their interactions are essential to development of a model-based approach for reducing motion artifacts in order to acquire real-time health information by WISEs.
V. APPLICATION: CAPTURING TRANSIENT CARDIOVASCULAR FEATURES DURING WATER DRINKING
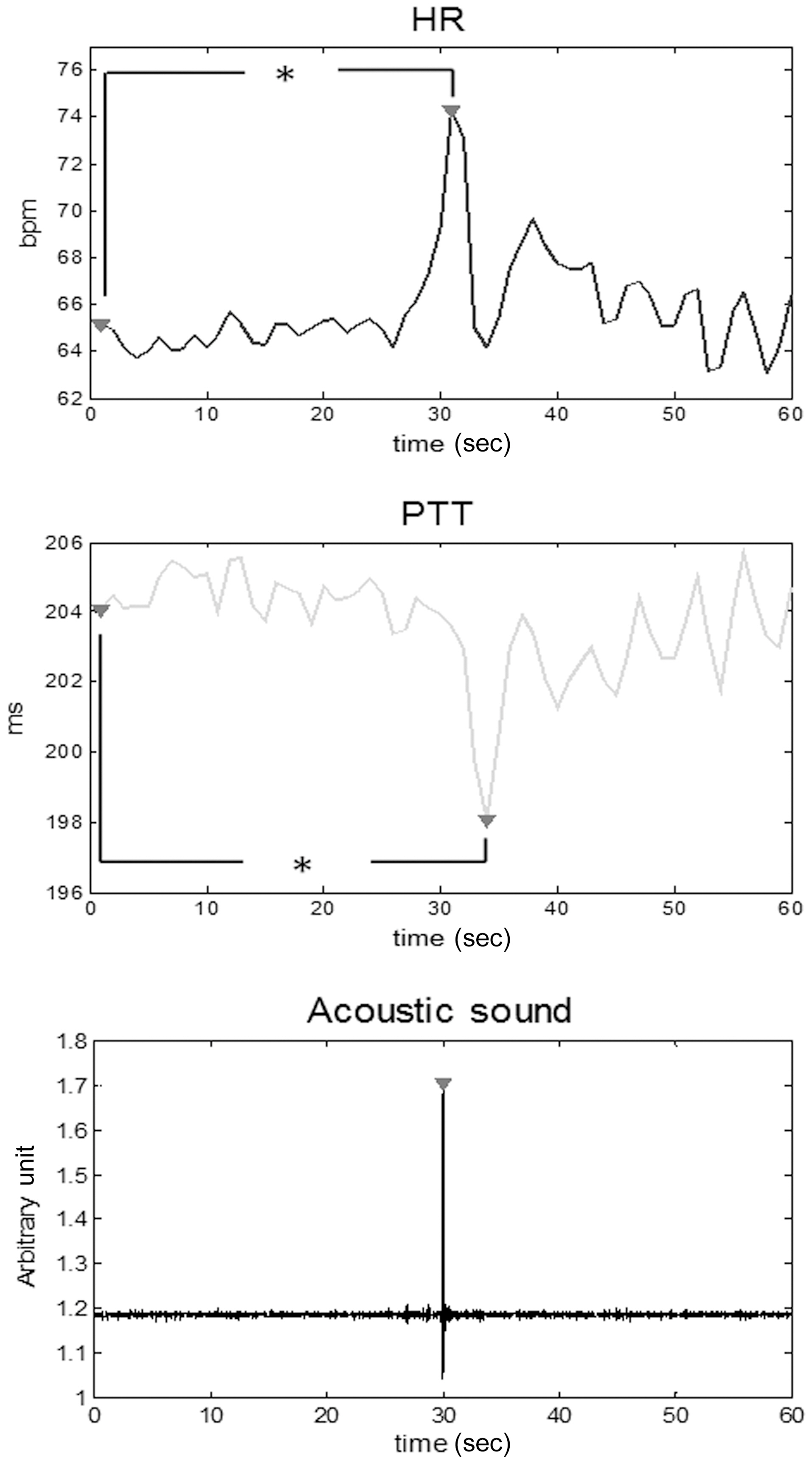
One of the biggest advantages of using WISEs is that they can supply transient features during daily activities such as swallowing. Swallowing disorders frequently occur in the elderly and monitoring during the swallowing process is therefore of interest for assistive living technologies for the elderly. In this study, preliminary results for continuous monitoring of HR and PTT, which is commonly acknowledged to be inversely related to BP, during water drinking are presented.
Seven healthy subjects (including 4 males and 3 females)participated in the experiment. The subjects were 26 ± 6 years old, 171 ± 8 cm tall and weighed 66 ± 7 kg. All subjects were non-smokers. The subjects were required to refrain from eating or drinking 2.5 hours before the experiment.
The experiment was conducted in the supine position. ECG and PPG data were collected from the subjects’ wrists and left index finger respectively using an in-house system. A microphone sensor was placed on the subjects’ throat to capture sound induced during the moment of swallowing. The signals were all sampled at 1 kHz.
Upon arrival, the subjects were asked to rest in the supine position for 10 minutes. They were then asked to drink water through a straw, by taking a single swallow once every minute on 5 occasions.
All data trials were performed on a beat-to-beat basis. The RR interval (RRI) was determined as the time lapse between the successive R peaks of ECG. PTT was measured as the time interval between the peaks of ECG R waves and the first derivative of PPG signals in the same cardiac circle. The HR was calculated from the RRI in beats per minute (bpm).
Swallows were characterized by finding peaks in the acoustic signals captured by the microphone. There were 5 datasets for each subject, each containing data for a single swallow. Data for a 30-second interval before and after each swallow was segmented. In total, 35 datasets, each for a 1 minute interval,were obtained. All the datasets were then interpolated to 1 Hz and averaged. The difference between parameters taken during swallowing (i.e., the peak value) and during the baseline interval (i.e., during the first second) was statistically analyzed by using the paired Student’s t-test. A P-value less than 0.001 is considered as statistically significant.
Fig. 5 plots the averaged response of HR and PTT induced by a single swallow for the 35 datasets. Significant and transient increases in HR and decreases in PTT were observed during swallowing during 30 seconds (P < 0.001). The standard deviation of HR and PTT during each second varies from 6.9 bpm to 10.5 bpm, and from 15.1 ms to 18.5 ms, respectively,before swallowing; and it subsequently varies from 8.4 bpm to 12.1 bpm and 14.6 ms to 18.1 ms.
Swallowing is a complex and highly coordinated activity involving muscles of the tongue, larynx, pharynx and esophagus[68]. Previous study has found chronotropic increase of HR
for a single swallow and a series of three or more swallows[68]. There was also a rapid and significant increase in BP during water drinking [69]. These simultaneous cardiovascular responses were generated by the feedback from the pharyngeal and esophageal receptors. A feedforward mechanism by a central descending signal from the higher brain centers may also contribute to these responses.
PTT is inversely related to BP by the Moens-Korteweg equation. In this study, a transient but pronounced decrease was observed in PTT in addition to an increase in HR, indicating that a transient increase in BP is induced by a single swallow.These abrupt responses could be attributed to inhibition of vagal activity during swallowing, since sympathetic signals cannot be transmitted over such a short interval (1-2 secs) due to the slow adrenergic synapses [68]. The influence of sympathetic activity on PTT needs to be further investigated by introducing a series of swallows rather than a single swallow.
Driven by an increasingly aging population, the prevalence of chronic diseases, and continuously rising healthcare costs, a new health paradigm that emphasizes early detection, early diagnosis and early treatment, is highly recommended. In this new paradigm, WISEs have been identified as crucial components to provide noninvasive and continuous monitoring to assist the lives of the elderly and other chronic patients. In the past few decades, the development of WISEs has experienced several major technological advancements, including emerging technology for e-textile sensors. Nevertheless, there are a number of technical challenges that remain to be solved for WISEs.This includes a complete solution to motion artifact reduction in real-life, real-time measurements.
The applications of wearable technologies are wide ranging.In this study, the latest measurement results of transient surges in HR and BP, as indicated by the reduction in PTT, during swallowing have been presented. The results indicate the possibility of monitoring swallowing in the elderly.
Another potentially viable application of WISEs will be to predict near-term risk of cardiovascular diseases, which is another chronic disease commonly found in the elderly. A considerable number of cardiovascular disease patients die suddenly without prior symptoms and therefore, wearable technologies can play a major role in identifying transient activity-related features to potentially predict near-term risks of cardiovascular events or deaths.






![Device prototypes in wearable intelligent systems for e-Health(WISEs) by different research groups. Clockwise from the upper right handcorner are: an earlobe photoplethysmogram (PPG) detector [7] a wristworndevice AMON for blood pressure (BP) electrocardiogram (ECG)blood oxygen saturation monitoring [8] a glove for monitoring skintemperature PPG and galvanic skin response [9] a respiration waist belt[10] a ring-type PPG sensor [11] a cuff-less BP watch [12] produced byJetfly Technology Ltd. Based on the technology developed in our researchcenter and an h-shirt developed in our research center for monitoringPPG ECG and BP [13].](http://oak.go.kr/repository/journal/10860/E1EIKI_2011_v5n3_246_f001.jpg)
![A wearable artificial endocrine pancreas with a needle-typeglucose sensor [28].](http://oak.go.kr/repository/journal/10860/E1EIKI_2011_v5n3_246_f002.jpg)
![An h-shirt-based wearable intelligent systems for e-Health(WISEs) prototype developed in our research center for monitoringelectrocardiogram photoplethysmogram and cuff-less blood pressurebased on the estimation of pulse transit time [13].](http://oak.go.kr/repository/journal/10860/E1EIKI_2011_v5n3_246_f003.jpg)
![Cluster of European Commission co-financed projects on smartfabrics interactive textiles reproduced from [49]. IST: Information SocietyTechnologies.](http://oak.go.kr/repository/journal/10860/E1EIKI_2011_v5n3_246_f004.jpg)