The brown seaweed (Laminariaceae)
Quantitative measurement of phlorotannins in seaweed tissues by colorimetric method has been uncertain (Stern et al., 1998) and crude phlorotannins have been measured simply on a dry weight basis (Shibata et al., 2004). In both methods, total amount of whole phlorotannins is measured. Such a total quantification of phlorotannins provides no information on the biological and pharmacological properties of individual phlorotannins. Indeed, phlorotannins are highly soluble in mixtures of water and organic solvent, and high-performance liquid chromatography (HPLC) can act as a suitable tool for their qualitative and quantitative analysis. Koivikko et al. (2007) used HPLC to analyze phlorotannin from
>
Collection of seaweed materials
The brown alga
The seaweed thalli were cleaned with filtered seawater. Epiphytes were removed by sonication for 1 min. The samples were then dried using different drying methods: sun-dried at room temperature for 3 days; shadow-dried at room temperature for 3 days with an electric fan; oven-dried at 60ºC for 24 h; and lyophilized for 24 h. For lyophilization, a vacuum freeze dryer (SFDSM 24L; Samwon Freezing Engineering Co., Busan, Korea) was used. To remove salt from the thalli, samples were washed with tap water. The mature blades of
>
Extraction of crude phlorotannins
Crude phlorotannins were extracted from the algal powder according to the method of Folch et al. (1957), with some modifications. The algal powder (0.5 g) was rotated on a rotator (Rototorqe 7637-10; Cole-Parmer, Vernon Hills, IL, USA) with methanol (2 mL) at room temperature for 2 h. Chloroform (4 mL) was added and the mixture was shaken for 5 min and filtered with defat-cotton. After filtration, the methanol-chloroform extract was partitioned by adding deionized water (1.5 mL) with shaking for 5 min. The upper layer (non lipid fraction) was collected and extracted by ethyl ether (3 mL). The ethyl ether fraction was then evaporated with a nitrogen generator (G 4510E; Domnick Hunter Ltd., Dukesway, England). The crude phlorotannin residue was collected and measured with a digital electronic balance (Mettler AG245; Toledo GmbH, Greifensee, Switzerland). The crude phlorotannin was dissolved in 100% methanol (1 mg/mL) and stored at -20°C until use.
>
Isolation of dieckol and phlorofucofuroeckol-A
To isolate the pure dieckol and phlorofucofuroeckol-A from the crude phlorotannin extracted from
>
Quantification of dieckol and phlorofucofuroeckol-A
To measure the amounts of dieckol and phlorofucofuroeckol-A from
All data are presented as the mean±SEM. Statistical comparisons of the mean values were performed by an analysis of variance (ANOVA), followed by a Duncan’s multiple test using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA).
orgsignificance. The results shown in each of the figures are representative of at least four independent experiments.
>
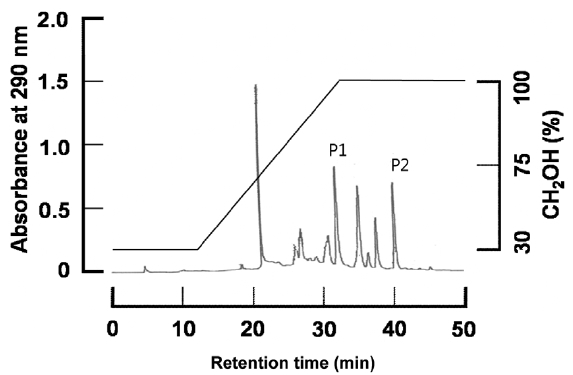
Validation of HPLC quantification
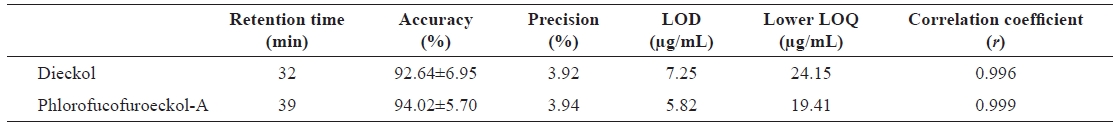
To quantitatively evaluate dieckol and phlorofucofuroeckol-A from the
>
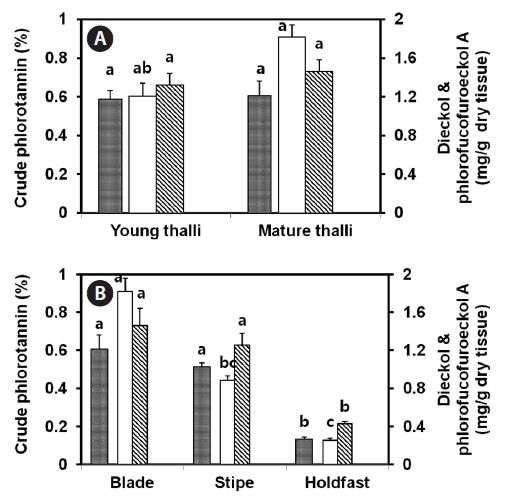
Distribution of phlorotannins in E. cava tissue
To determine the chemical distribution of phlorotannins in different tissue parts of
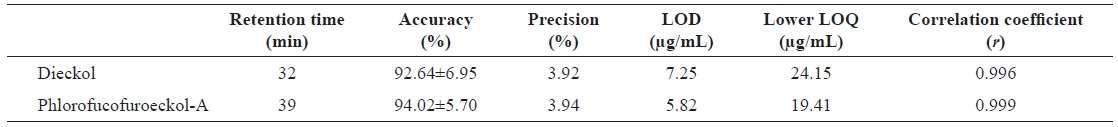
Validation parameters for the determination of phlorotannin compounds in Ecklonia cava extract
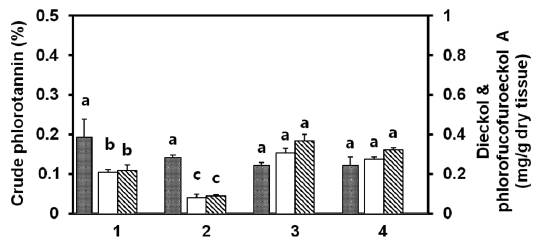
compared for the amounts of crude phlorotannin, dieckol, and phlorofucofuroeckol-A. Blade, stipe, and holdfast from mature thalli were also compared with regard to the amounts of phlorotannins. All samples were lyophilized. As compared with young thalli, mature thalli contained 1.5-fold more dieckol (1.82 mg/g-dry tissue). In the case of crude phlorotannin and phlorofucofuroeckol-A, mature thalli showed marginally higher amounts than young thalli (Fig.2 ). Among the blade, stipe, and holdfast of mature thalli, the blade portion generally showded the highest amount of crude phlorotannins (0.61±0.07%), dieckol (1.82±0.12 mg/g-dry tissue), and phlorofucofuroeckol-A (1.46±0.18 mg/g-dry tissue). The sum of these two compounds represented about 54% of the crude phlorotannins. The holdfast contained a low level of phlorotannins.
>
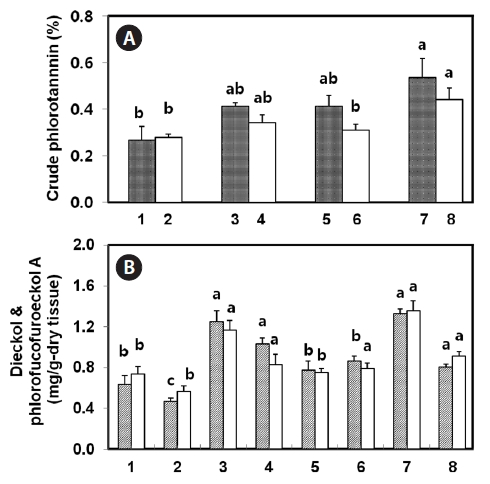
Drying pretreatments on phlorotannin extraction
ments, such as sun-drying, shadow-drying, oven-drying, and lyophilization, of both natural and tap water-washed thalli, were analyzed to compare different drying methods with regard to the extraction of phlorotannins. Among the samples, natural non-washed lyophilized tissue yielded the highest amount of crude phlorotannin (0.53%) on a dry weight basis, whereas sun-dried tissues showed the lowest yield (0.27%). Overall, tap water-washed tissue was found to have lower crude phlorotannin than non-washed tissue (Fig.3 A). Compared with natural tissues for crude phlorotannin, shadow- and oven-dried tissues were extracted at almost 80% of lyophilized tissue. Meanwhile, sun-dried tissues of both natural and tap water-washed thalli showed only half the yield of lyophilized tissue. When considered dieckol and phlorofucofuroeckol-A contents in
was observed between lyophilized and shadow-dry tissues of both natural and salt-removed thalli (Fig.3 B). Approximately 90% or more dieckol and phlorofucofuroeckol-A was extracted from shadow-dried tissue as compared with lyophilized tissue. In the case of sun-dried and oven-dried thalli, approximately 60% of the phlorotannins were extracted. Washing with fresh water decreased the compounds of compounds extracted from thalli down to 61-82% of that from natural tissues, even when shadow-dried or lyophilized. In the case of natural non-washed lyophilized and shadow dried tissues, the sum of dieckol and phlorofucofuroeckol-A represented 51% and 59% of the crude phlorotannins, respectively.
>
Effect of boiling and steaming on phlorotannin yield
Boiling or steaming before tissue drying and extraction is commonly used to denature enzymes that are involved in the decomposition of useful compounds. To determine the effects of boiling and steaming before drying pretreatment on the yield of phlorotannins from
Marine brown algae have recently become a subject of interest due to their bioactive secondary metabolites, such as phlorotannins.
More amount of crude phlorotannin was present in mature thalli as compared with young thalli (Fig.2 ). Similar to other phenolic compounds, phlorotannins are produced extensively in the cytoplasm or chloroplasts of growing tissues, and are subsequently stored in physodes (Schoenwaelder and Clayton, 1998). Generally, young thalli produce more primary metabolites with faster growth, whereas mature thalli have multiple mechanisms to modify biomolecules, thus supporting diverse cellular functions (Chen et al., 1998). Phlorotannin-rich cells were found to be localized only within the outer cortical layer of the blades of
In this study, four dry pretreatments were applied. Among them, approximately 90% or more dieckol and phlorofucofuroeckol-A could be extracted from shadow-dried tissue as compared with lyophilized tissue, whereas sun-dried thalli yielded the lowest amount. Lyophilization or cryodesiccation are processes used for the dehydration of almost all heat-sensitive materials. The materials are frozen and then the surrounding pressure is reduced. Just enough heat is then added to allow the frozen water in the material to sublime directly from the solid to the gas phase (Oetgen and Haseley, 2004). However, the equipment and operating costs for freeze-drying are higher, and the drying capacity is lower, than for sun-, shadow-, or oven-drying. Loss of phlorotannins during sun-drying might be due to photo-oxidation processes. Some phenolic compounds are decomposed rapidly when exposed to direct sunlight or dried at elevated temperature (Mueller-Harvey, 2001). Oven-drying at 60°C may not immediately inactivate degradative enzymes, and thus phlorotannin compounds may be degraded before complete drying. Washing, boiling, and steaming procedures with fresh water likely released some water-soluble phlorotannins from tissues. Considerable amounts of phlorotannins were observed in the water after boiling or even dipping in seawater at room temperature (data not shown). One explanation could be the presence of certain very polar phlorotannins in