The pandalid shrimp Pandalus gracilis Stimpson 1860 is widely distributed from temperate to tropical regions in the Northern and Southern hemispheres,and is an important target in world fisheries (Holthuis, 1980). It represents an important traditional resource in the sea off the Korea Strait and the Tsugaru Strait,Sendai Bay, Japan, and dominates the prawn catch off Korea (Yoshida, 1941; Komai, 1999; Oh et al., 2008).
The genus Pandalus contains 19 species, which are found at all depths on the continental shelf and slope in the Northern Hemisphere. Members of this genus are of commercial importance and scientific interest because their reproductive patterns show stronger sexual dimorphisms in the structure and armament of the third and fourth pereopods compared to any other species in the group. Most pandalid shrimps are protandric hermaphrodites, becoming mature females after spending 1-4 years as functional males, depending on the species and geographic location (Butler, 1964; Holthuis, 1980; Hayashi, 1988; Komai,1999; Bergstrom, 2000), and they have been used as model organisms for studying the evolution of sex change (Charnov, 1982; Bergstrom, 2000). The timing of their sex change often fluctuates among years and populations, and as result, both early- and late-reversing individuals occur in these populations (Bergstrom, 2000). Also, the distribution of pandalids is related to their reproductive biology and the specific ecosystem. They play an important role in coastal food webs, both as predators feeding on a variety of foods ranging from diatoms, copepods, and small mollusks to detritus, and as prey for many fishes, marine mammals, and other predators (Butler,1980; Berenboim, 1981).
Despite its ecological and economic importance,few studies have examined the biological and ecological aspects of P. gracilis. Studies have described its reproductive biology in the southeastern coastal waters of Korea (Oh et al., 2008), larval development in the laboratory for species identification (Lee et al., 2007), and larval survival rates and growth under two temperature conditions for aquaculture (Choi and Ma, 2004). Its specific status was synonymous (Holthuis, 1980; Hayashi, 1988) and recent new occurrences have been reported (Li, 2006, 2007).
Recently, Bergstrom (2000) published a comprehensive review on the biological aspects of Pandalus species. Hayashi (1988), however, revived the species P. gracilis based on four morphological characteristics from new material. Thus, only a few investigations have studied the reproductive and ecological aspects of this revived species. The present paper describes the stage of sex transition, differences between male and female populations, and sexual differences in growth on the basis of monthly lengthfrequency data analysis (LFDA).
Monthly samples of P. gracilis were taken between May 1998 and April 2000 from the southeast coast of Korea (34°30′ N, 128°40′ E) (Fig. 1). Sampling was conducted down to 25 m below chart datum using a beam trawl. Samples were fixed in 4% neutralized formalin for 1 day and then transferred to 70% alcohol for storage. The seawater temperature of the bottom layer in the sampling area was recorded.
Sex was determined by the shape of the endopodite of the first pleopods and the presence or absence of the masculine appendix of the second pleopods.
Based on morphological characteristics of the endopod of the second pleopods, transitional stages between male and female phases were identified to determine whether P. gracilis is a protandrous hermaphrodite. Carapace length (CL), the shortest distance between the posterior margin of the orbit and the middorsal posterior edge of the carapace was measured using a dissecting microscope (Zeiss Stemi SV-6; Jena, Germany) and Image-Pro Plus version 4.1.
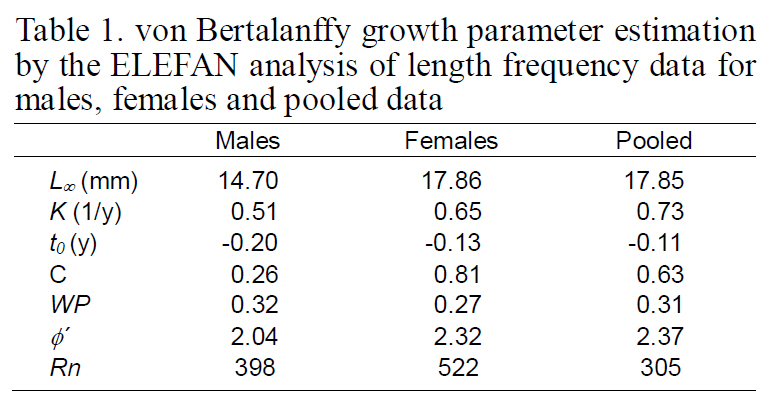
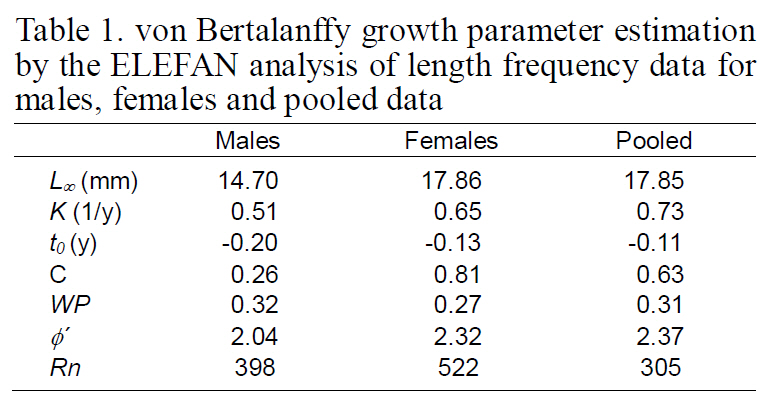
Length-frequency distributions (LFDs) were constructed for males, females, and pooled data using 1 mm intervals of CL. Growth was described using the modified von Bertalanffy growth function (VBGF) (Pauly and Gaschutz, 1979):
where L∞ (L infinity) is the asymptotic length, K is the intrinsic growth rate, t0 is a theoretical age at zero length, C is the amplitude of seasonal growth oscillations, ts is the age at the beginning of the growth oscillation, and WP (=ts+0.5) is the time of year when growth is slowest.
Growth curves were estimated from the LFDA using the program ELEFAN in FiSAT (Gayanilo et al., 1997), which uses a nonparametric method to fit the modified VBGF through modes. The Rn value gives an estimator of the goodness of fit. ELEFAN estimates the growth parameters (L∞, K, C, and WP)without SEs. According to Pauly (1987), t0 estimates cannot be obtained solely from the length-frequency data, and therefore, supplying a CL at first zoeal hatching of 1.4 mm CL (Lee, 2003) to the ELEFAN program was necessary to allow estimation of t0.
Growth performance was compared using a growth performance index (φ') (Pauly and Munro, 1984):
A chi-square test was used to test for differences in the sex ratio. Differences in the size-frequency distributions of the population between the 2 sampling years and the size-frequency distributions of the population between sexes were determined using a Kolmogorov-Smirnov two-sample test (Sokal and Rohlf, 1995). Statistical correlation analysis of Pearson product moments was accomplished using MINITAB 15. Kimura’s likelihood ratio test (Haddon, 2001) was used to investigate differences in growth between the sexes.
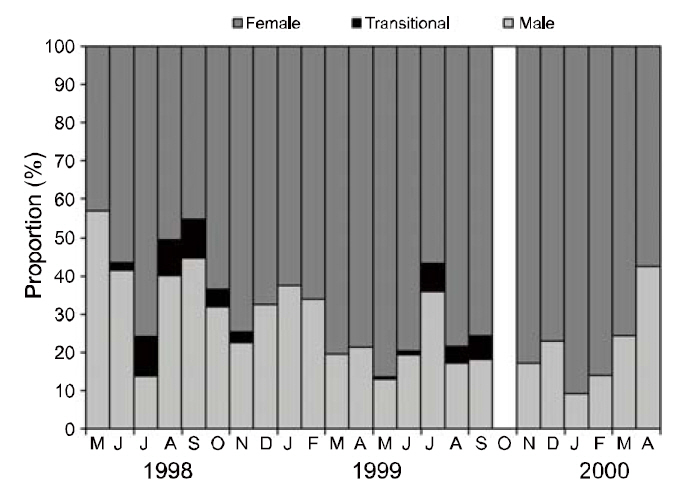
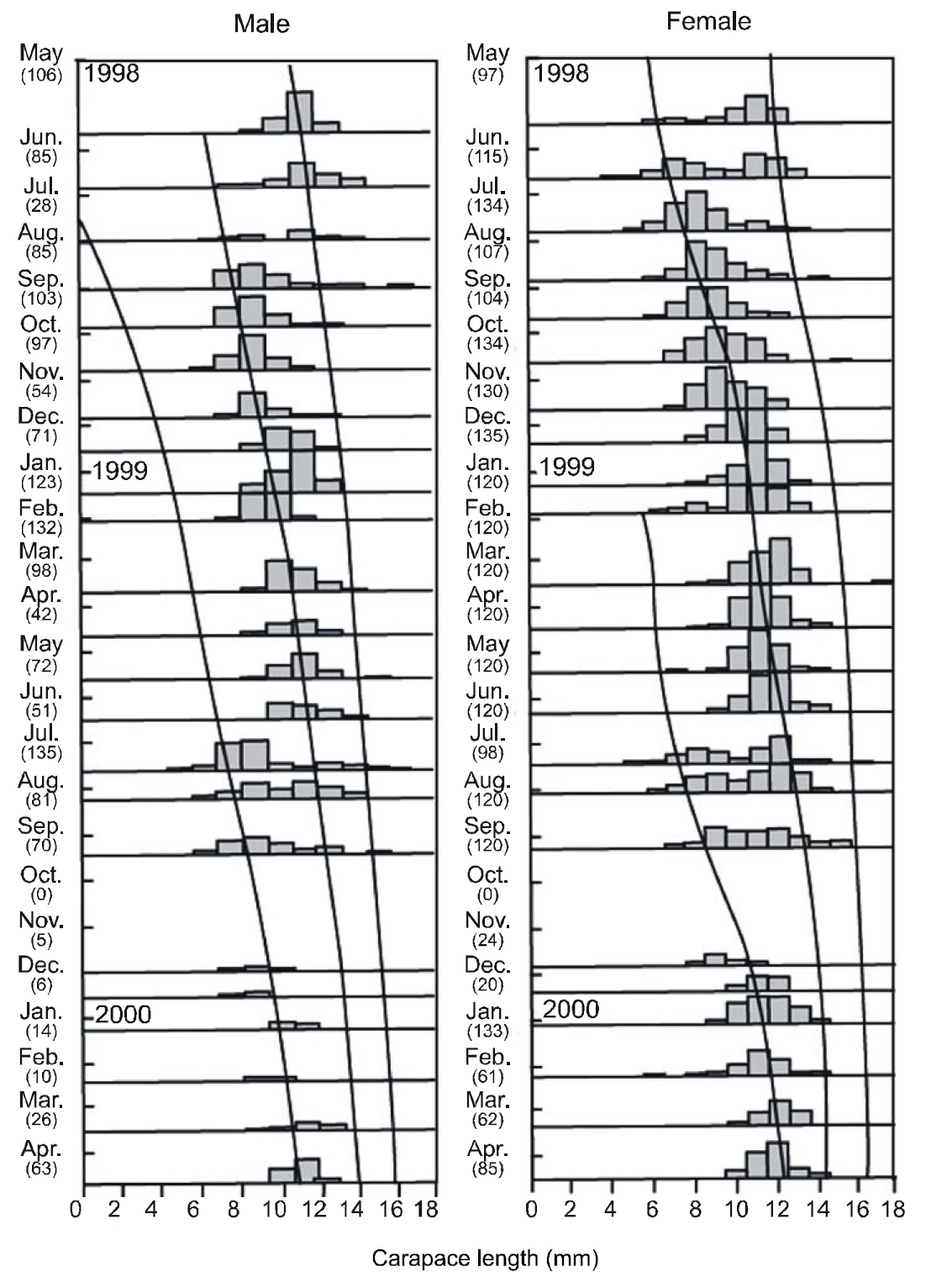
All specimens of P. gracilis sampled from May 1998 to April 2000 were sexed during each month.Shrimp samples were not collected in October 1999.The shrimp beam trawl (rigid beam length: 7 m), with a mesh size of 0.98×0.98-mm, was dragged horizontally. Trawl duration ranged from 20 to 90 min at speeds of 0.4-2.0 knots. Of the 4,127 specimens collected, 57% were identified as females,39% were males, and 4% were transitional stages (Fig. 1). The number of females was significantly greater than that of males and transitionals throughout the sampling period (χ 2=1,520.15, P<0.001), although the numbers of females, transitionals, and males varied slightly among months and years (Fig.2). Transitional individuals of P. gracilis mainly occurred in the summer. Temperature ranged from 8.46°C in February 2000 to 24.38°C in August 1998. A significant correlation was observed between the number of transitionals and ambient seawater temperature (r=0.886, P<0.001).
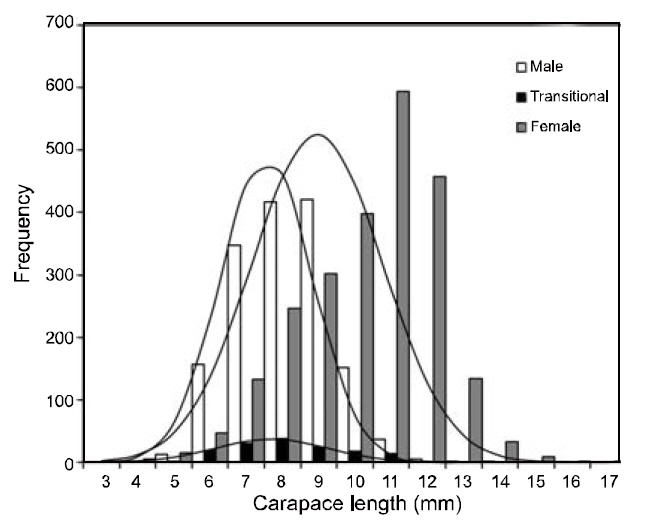
Between May 1998 and April 2000, 4,093 individuals (1,557 females and 2,536 males) collected from the study area were used for growth estimations. CL length ranged from 3.16 mm to 16.91 mm. These lengths were subdivided into 1 mm CL size classes prior to growth analysis. The LFDs showed that the population had two modal size groups per year, with similar patterns in both sexes. Apparent shifts were seen in the modal length of cohorts with time. In both sexes, small newly recruited shrimp appeared during the period between June and August. This pulse could be followed until September 1999, and disappeared in October 1999. The best fit to the length-frequency data identified a distinct new cohort, which became apparent in June or July in each year.
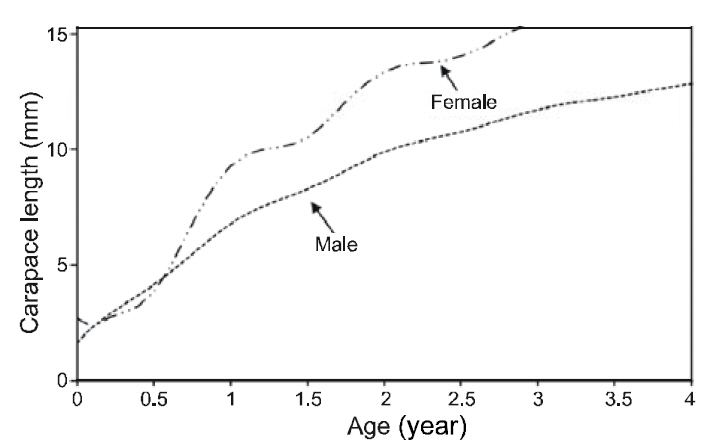
The VBGF parameters estimated by ELEFAN for each sex and for pooled data are summarized in Table 1. The values of L∞ and K for the pooled data were 17.85 mm CL and 0.73/y, respectively. Analyses of the modal progression for each sex separately showed that females (L∞=17.86-mm CL, K=0.65/y) were higher in both L∞ and K than males (L∞=14.70 mm CL, K=0.51/y) (Fig. 3). Females had faster growth rates than males, which was also indicated by the growth performance indices (φ´) of 2.04 for males and 2.32 for females (Table 1). Kimura’s likelihood ratio test showed significant differences in L∞ (df =1, χ 2=7.82, P<0.01) and K (df =1, χ 2=4.56, P<0.05), but no difference was observed in t0 (df =1, χ 2=2.54, P>0.05) between the sexes. A significant difference in the growth curve was detected between sexes(df =3, χ 2=8.74, P<0.05).
The growth curve showed strong seasonal oscillations of 26% for males, 81% for females, and 63% for the combined data. The slow-growth phase occurred in April for males (WP=0.32), females(WP=0.27), and the pooled data (WP=0.31) (Fig. 4 and Fig. 5). Mean size and age (95% confidence limit) at sex transition, calculated from the growth parameters, were 7.07 mm (±0.27) CL and 1.05(±0.21) years, respectively.
In this study, females had faster growth rates than males, which indicates differences in growth patterns between the sexes. Similar results were found in Pandalus borealis and Pandalus jordani (Allen,1959; Hopkins and Nilssen, 1990; Bergstrom, 1992).Because of the sex-differentiated growth pattern,Bergstrom (1992) suggested that growth in sequential hermaphrodites may be best described by either separate models for each sex, or perhaps for detailed growth analysis, by a model that combines the growth of each sex in relation to their proportional occurrence in the population. Mean size and age at sex transition were 7.07-mm (±0.27) CL and 1.05(±0.21) years, respectively, suggesting that P. gracilis reached a transitional stage at 1 year of age. Considering the life span of this species (4.7 years), calculated from the mean CL of the larger size group and the growth parameters, P. gracilis seems to change sex at an early life stage. Differences in size and age at sex transition exist between P. gracilis and other pandalid shrimps (Bergstrom, 2000), which is likely associated with their reproductive strategies.
Fished species may be dioecious or hermaphroditic.Sexual reproduction can provide an advantage because it creates and perpetuates genetic diversity and allows the formation of novel genetic combinations that provide adaptations to ever-changing environments (Williams, 1975; Bell, 1982; Michod and Levin, 1988). All elasmobranchs have separate sexes throughout their lives and population sex ratios are usually close to 1:1. Most temperate teleost fishes are dioecious, but almost 50% of fished families in the tropics contain hermaphroditic species (Sadovy,1996). Sequential hermaphrodites may be protogynous,in which adult females change to adult males, or protandrous, whereby adult males change to adult females. The fished families Lethrinidae, Scaridae, Labridae, Sparidae, and Serranidae contain protogynous species. Protandrous hermaphroditism has been observed in Sparidae, Centropomidae, and Platycephalidae.
The sex ratios of hermaphroditic species are affected by fishing because they can be size-related.In protogynous species, for example, males are larger than females; therefore size-selective fishing leads to a female-dominated population. Why does sex change occur? Selection should favor sex changes with increasing size or age when one sex gains a relatively greater reproductive advantage with size or age (Warner, 1975, 1988; Charnov, 1993; Reynolds, 1996). Thus, if male reproductive advantage increases faster than that of females, a female to male change will be favored. Conversely, if female reproductive advantage increases faster than that of males, then a male to female change will be favored. For species that change sex from male to female, a lower disparity between the sexes in sexual selection exists due to dispersed breeding resources, which cannot easily be monopolized by a single male.
The rate of gain with size or age is therefore slower for males and is exceeded by the gains that females receive per unit size due to fecundity advantages. The initial sex is always more abundant in fishes that change sex, resulting in a male bias for protandrous species and a female bias for protogynous species (Choat and Robertson, 1975). Some decapod crustaceans, such as pandalid and crangonid shrimps,which are commercially exploited, are protandrous hermaphrodites (Tiews, 1970; Bergstrom, 2000). These shrimps exhibit a wide range of reproductive strategies because of the characteristics of sex transition.
In this study, a significant correlation was observed between seawater temperature and the number of transitionals, which indicates that the rate of sex transition from male to female can be affected by temperature. Previous studies suggested that several factors affected sex changes in pandalid shrimps (Koeller et al., 2000; Chiba et al., 2003; Wieland, 2004; Skuladottir et al., 2005). According to Wieland (2004) and Skuladottir et al. (2005), the change in size at sex transition in P. borealis was significantly correlated with temperature. Our study is consistent with their results. Koeller et al. (2000) noted that size at sex transition in P. borealis was inversely related to female density, emphasizing that density was the most important factor determining individual growth at a high population density. Adding another factor,Chiba et al. (2003) stressed that male-male com
petition leads to a delayed sex change.
In conclusion, P. gracilis is a protandrous hermaphrodite. As individuals grow, the female composition in the population becomes predominant.The rate of sex transition in this species can be affected by seawater temperature, suggesting that annual changes in the sex ratio of the population have serious effects on population dynamics and subsequently affect the annual catch of the stock (Food and Agriculture Organization of the United Nations,2007). Thus, management strategies for the stock need to be applied differently, with detailed information on the effect of temperature and density on the rate of sex transition. Further studies will focus on the effects of several factors that determine sex transition on population dynamics.