Groupers of the genus
Currently, seed for grouper species were obtained through hormonal treatment of broodstock or by collection of fingerlings from the wild. Obtaining males from the wild for spawning is problematic because groupers are protogynous hermaphrodites(Smith, 1965; Tan and Tan, 1974; Chen et al., 1980;Shapiro, 1987; Brusle-Sicard et al., 1992; Shapiro et al., 1993; Sadovy and Colin, 1995). Obtaining seed stock can be time-consuming, economically prohibittive,or unfeasible due to the late puberty of females,the extremely long time period required for sex inversion, and the rarity of males in the wild.Therefore, induction of precocious puberty and sex inversion is very important for commercial aquaculture purposes (Sarter et al., 2006).
Mature female broodstock can be readily obtained from captive stock, but simultaneous availability of mature male broodstock is the most severe constraint to artificial propagation. However, several research groups have attempted induced spawning in groupers through hormonal manipulation (Chen et al., 1977;Chao and Lim, 1991). In
Among synthetic steroids, MT effectively enhances growth in fish. One distinct advantage of anabolic steroids over growth hormones is that they can be provided in food without losing their biological activity. Androgens are mainly produced by the testes and possess anabolic properties for promoting growth of sex-related tissues and of the whole animal.Generally, androgens are more effective than estrogens in promoting growth in fish (Weatherley and Gill, 1987). McBride and Fagerlund (1973)examined the effect of food-administered MT on growth of juvenile coho salmon
In April 2009, 1-year-old fish were obtained from the Gyeongsangnam-do Fisheries Resources Research Institute and were transported to and maintained at the Fishery Genetics and Breeding Science Laboratory, Korea Maritime University,Korea. The fish were divided into eight experimental groups: a sham control group injected over 8 weeks(A), a group injected with 17 α-methyltestosterone(MT; Sigma, St. Louis, MO, USA) over 4 weeks (B),a group injected with MT over 8 weeks (C), a group orally provided 1 kg of feed with 0.5 mg of MT (E), a group given 1 kg of feed with 1.0 mg of MT (F), a group given 1 kg of feed with 2.0 mg of MT (G), and two control groups (D, H). Each experimental group included 30 fish. Fish were reared for 14 weeks in seawater tanks maintained at 24°C.
Fish were injected intramuscularly into the tissue behind the first dorsal fin at a dose of 0 (shaminjected control group) or 1 mg MT per kg of body mass. Injections were prepared from a mixture of coconut butter and 95% ethanol (Sigma) at a ratio of 1:9 and the total dose of MT. Fish were fed commercial extruded pellets (Aller Aqua Co. Ltd.,Aller, Denmark) containing 46.03% crude protein and 16.58% crude lipid two times per day, totaling 2% of the average body weight during the experimental period. MT feed was prepared by spraying 1 kg of feed with 0.5, 1.0, or 2.0 mg of MT dissolved in 50 mL of 95% ethanol (Howerton et al., 1992; Kuwaye et al., 1993). Following evaporation of the ethanol,the feed was stored at -20°C. Fish were fed two times daily, totaling 2% of the average body weight during the experimental period; the experiment was performed with three replicates.
At the start and end of the experiment, fish were weighed to the nearest 0.01-g using an electronic balance (Acom JW-1, Pocheon, Korea), and their standard length (LS) was measured to the nearest 0.01 cm using digital calipers (Mitutoyo CD-20CP,Kawasaki, Japan). Using these data, we estimated the growth rate for body weight (GRW), specific growth rate (SGR), condition factor (CF), and feed efficiency ratio (FER). Differences among groups were analyzed using analysis of variance (ANOVA) in SPSS ver. 9.0 (SPSS Inc., Chicago, IL, USA), and multiple comparisons were performed using Duncan’s multiple range test (Duncan, 1955).
For histological analysis, gonads were removed and tissue samples were fixed in 10% neutral formalin solution (100 mL formalin, 6.5 g Na2HPO4·12H2O, 4.5 g KH2PO4, 900 mL DW) for 5 days. The samples were then re-fixed in Bouin’s solution for 24 h. Samples were prepared in 6-㎛-thick paraffin sections, placed on slides, stained with hematoxylin and eosin-phloxine B, and observed under a microscope(Axioskop; Zeiss, Oberkochen, Germany).Photographs of tissues were also taken.
Several methods of hormone administration for sex inversion in fish have previously been tested, including oral administration, immersion, intramuscular injection, and implantation (Hunter and Donaldson,1983; Pandian and Sheela, 1995; Beardmore et al.,2001; Piferrer, 2001). Table 1 presents data for
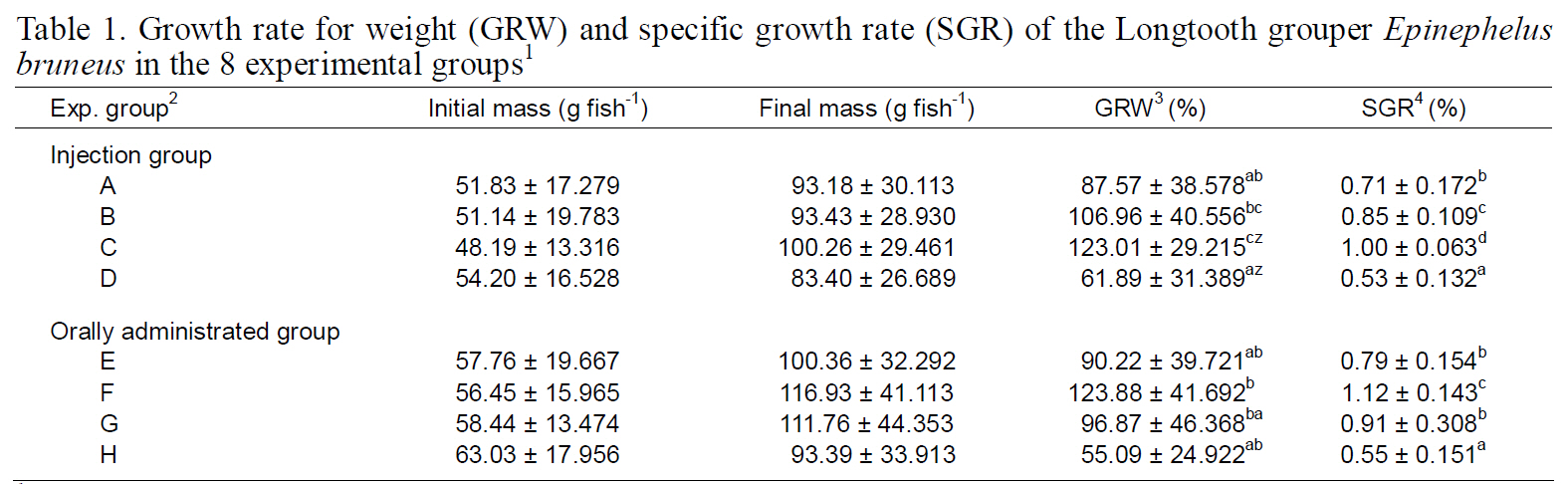
Growth rate for weight (GRW) and specific growth rate (SGR) of the Longtooth grouper Epinephelus bruneus in the 8 experimental groups1
GRW and SGR of longtooth grouper in the various experimental groups. Survival rate in all experimental groups was 100% and was not affected by the MT treatments.
GRW and SGR of the group injected over 8 weeks(C) were significantly higher than those of the shaminjected control group over 8 weeks (A), the group injected over 4 weeks (B), and control group (D)(P<0.05; Table 1). In fish orally provided MT, the GRW of the group provided 1 kg of feed with 1.0 mg of MT (F) was the highest among the examined groups. SGR of the F group was also significantly higher than those of the other groups (P<0.05; Table 1).
Data for the CF and FER of the longtooth grouper are provided in Table 2. CF of the group injected over 4 weeks (B) was higher than those of A, C, and D groups. FER of groups A, B, and C did not significantly differ from one another, but they did differ from the control group D (P<0.05). CFs of the groups given 1 kg of feed with MT (E, F, and G) were significantly higher than that of the control group (H).The experimental groups significantly differed in FER. FER of group F was significantly higher than those of the other groups (P<0.05; Table 2).
MT affected the growth of individuals that were given the hormone compared to those receiving no hormone. MT significantly enhances the growth rates of goldfish
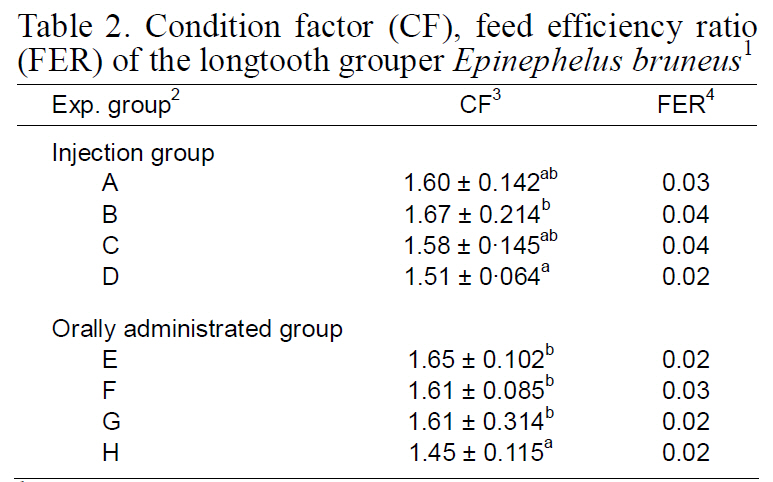
Condition factor (CF) feed efficiency ratio(FER) of the longtooth grouper Epinephelus bruneus1
ment increased the growth rate of longtooth grouper in our study. Therefore, we were able to determine not only the effect of MT treatment on fish growth,
but also a more effective dose of MT treatment. However,we were unable to determine the most effective dose, duration, or method of MT administration for longtooth grouper.
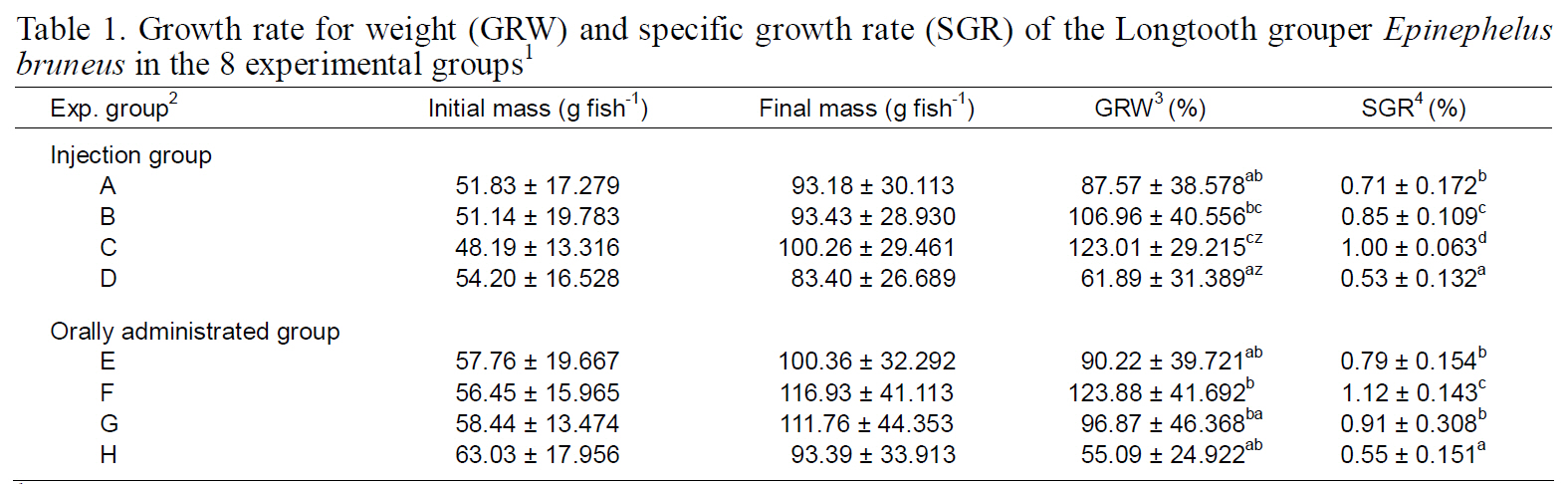
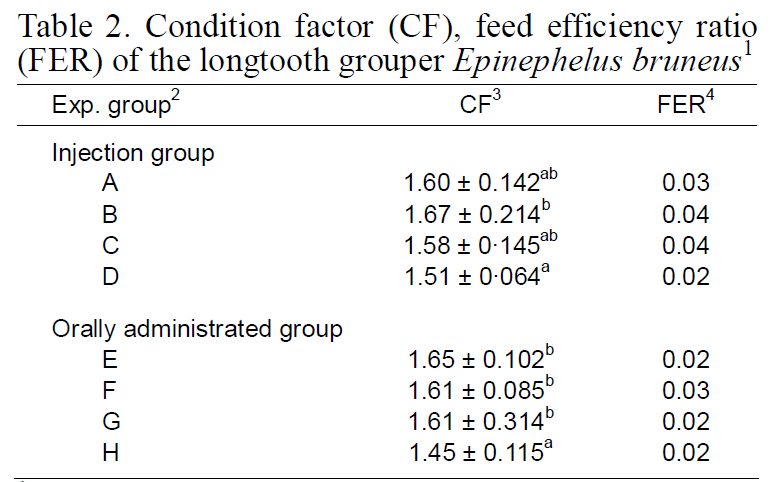
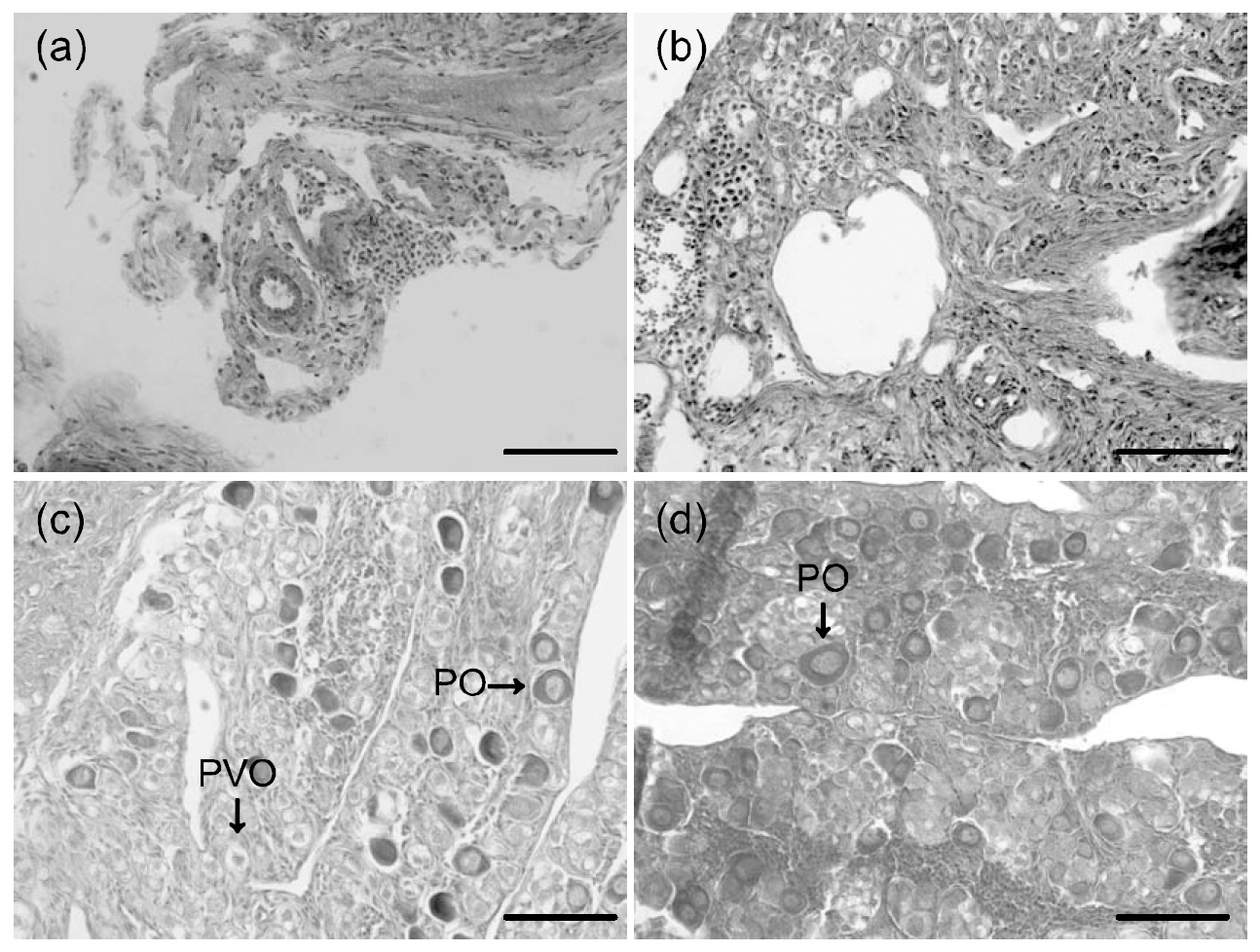
Gonadal histology of each group is illustrated in Fig. 1. Initially, fish exhibited mostly unidentified germ cell cysts (Fig. 1a). All fish in groups A, D, and H had unidentified germ cell cysts (Fig. 1b). In addition, fish in groups B, C, E, F, and G had previtellogenic and primary oocytes in the ovaries (Fig.1c, d). No spermazoa were present in the fish of any group. We did not obtain induced sex-changed males.
Sex change in longtooth grouper induced by androgen administration is essential for accelerating the reproductive cycle and for larval rearing. Yeh et al.(2003a) obtained 66.7% sex-changed males after a 70-day implantation in