Photopolymer has been researched and developed for semi-conductor applications. It is usually used as coating materials in industrial fields. There are three types of photopolymers - liquid, liquid crystal, and dry film. Photopolymer consists of monomer, sensitizer, initiator, and binder. The monomer has a functional group such as acrylate or epoxy. The initiator has sensitivity for a specific wavelength,so the wavelength of the laser can be selected by changing initiator and sensitizer. The composition of photopolymer should be optimized to get high sensitivity and diffraction efficiency [1-8]. Recently, there have been a lot of efforts to save and store more information [9-10].For this purpose, there has been much research to develop hologram storage materials and tools. The photopolymers are achieving the spotlight in the holographic field. They can reduce the cost of production. The synthesis of photopolymer is not too difficult and they are more cost effective than inorganic materials. Holograms are widely used in paper money, gift certificates, securities, and in many applications for holographic security media systems.
To satisfy these interests, many kinds of photopolymer which have different functional groups have been studied[14-19]. Holographic recording results from interference of the light in photopolymer film. During the recording, monomers diffused in the media and thus optical properties such as the refractive index of the photopolymer film are changed.By adding fluorescent polymer in the photopolymer film,the color and fluorescent intensity are changed. Using these properties, dual security systems can be possible[20]. Herein we report properties of photopolymer film containing fluorescent polymer and its applications for holographic recording and security film.
Ethylene glycol phenyl ether acrylate (PA) and polysulfone (PES, Mw= 35,000) were purchased from Aldrich. The triazine dimethacrylate (DT) was synthesized from cyanuric chloride, according to the literature [11]. Bis (μ5?2,4 cyclopentadien-1-yl)-bis-[2,6-difluoro-3-(1Hpyrrol-1-yl) phenyl] titanium(Irgacure 784) was purchased from Ciba Geigy and used as a photo-radical initiator. PMAn (polymethylanthracene,Mw= 3650) used as fluorescent polymer was synthesized using a reported procedure [21]. The solvent chloroform and 1, 1, 2, 2-tetrachloroethane (TCE) were purchased from Aldrich.
2.2. Preparation of photopolymer films
The photosensitive compositions consisted of a mixture of monomers (40 wt%), polysulfone (60 wt%), a photoinitiator and PMAn. PA is a monofunctional monomer and DT is a difunctional monomer. Polysulfone (0.75 g, Mw=35,000) was dissolved in chloroform and TCE. The polysulfone solution was a mixture of 0.5 g of monomers,0.013 g of Irgacure 784 and 2 wt% of PMAn in a brown bottle. Because of the Irgacure 784, the solution color is yellow. The yellow mixture was stirred at 150 rpm for 3 h with a magnetic stirrer, and filtered using membranes of 0.45 ㎛ pore size attached to a Teflon syringe. The solution was coated on a slide glass by spin coating. The films were prepared with different PMAn concentrations of thickness ~50 ㎛ and dried for 6 h.
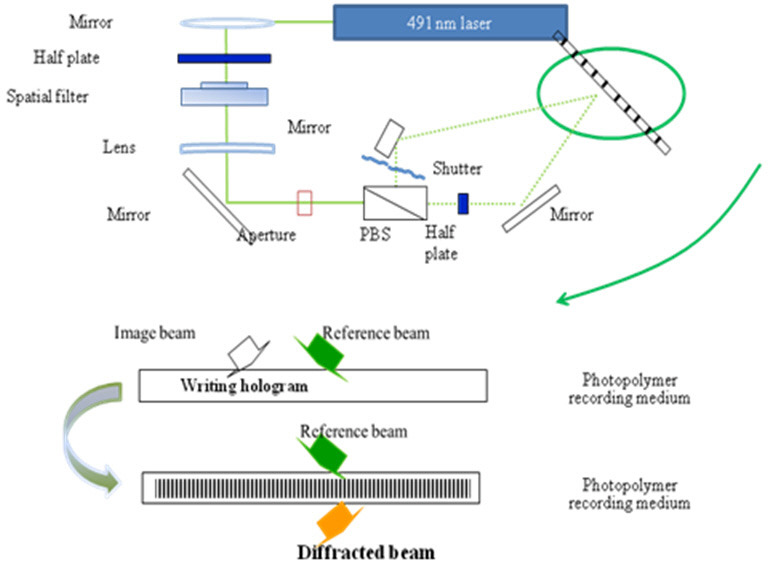
Holographic recording was generated by a series optical setup consisting of a diode laser (491 nm), polarizer, beam splitter, and detector, as reported before (Fig. 1) [14]. A power ratio of the object and reference beams was fixed as 1:1 by the adjustable polarized beam-splitter (PBS). The two beams were coherent on the photopolymer films and the interference pattern was generated at the films. The diffraction efficiency (DE) was determined from the ratio of the diffraction intensity (ID) against the sum of ID and intensity of transmission beam (IT). The blocking time of
the electrical shutters to measure diffraction efficiency of the photopolymer was programmed to be 0.5 second.
3.1. Diffraction efficiency of photopolymer film containing fluorescent material
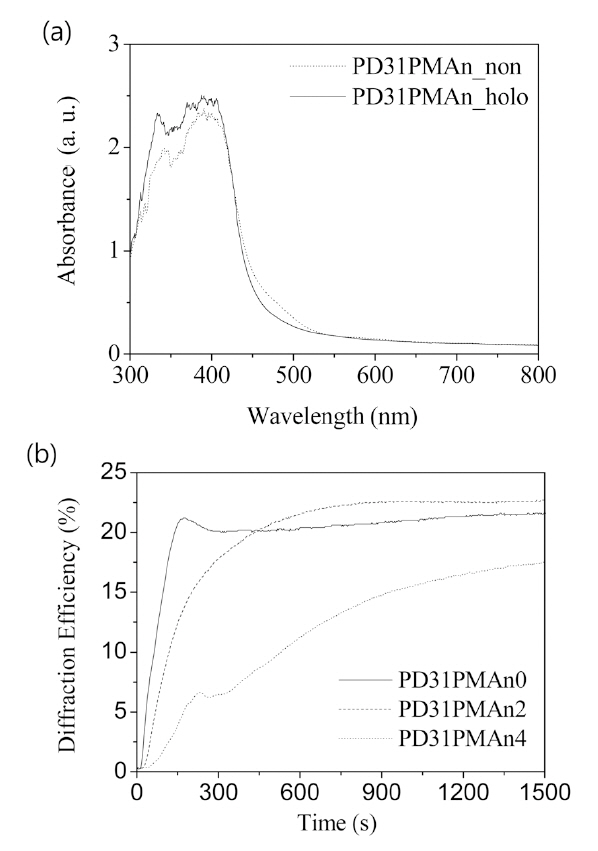
The yellow color photopolymer films which containing fluorescent polymer PMAn were prepared. Absorption spectra of this photopolymer film showed high absorption around 490 nm (Fig. 2a). This is due to Irgacure 784 absorption.Thus the absorption spectra were independent of PMAn concentration. The absorption at the 490 nm region makes holographic recording feasible on this photopolymer film at 491 nm. PMAn was stable during holographic recording[15-16].
The diffraction efficiency (DE) growth for the photopolymer film containing different PMAn concentrations is shown in Fig. 2 (b). The photopolymer film without PMAn was employed as a reference which showed maximum 22% of DE. The photopolymer film containing 2 wt% PMAn showed the highest DE among the samples. As PMAn concentration was increased over 2 wt%, DE decreased and the growth rate of DE was also reduced. These results could be ascribed to PMAn, which has a large enough size to disturb the diffusion as well as polymerization of monomers during the holographic recording.
3.2. Polymerization time by photo DSC
To examine the effect of PMAn on the polymerization of monomers in the photopolymer, we determined the polymerization time in the presence of PMAn by a photo DSC.The photopolymer solution was dropped on an aluminum substrate and dried [11]. Diffusion time was determined from the diffusion model according to the equation (Eq. (1)).
Where φ stands for the volume fractions of the different compounds; with φ(m) and φ(p) standing for monomer and polymer, respectively;
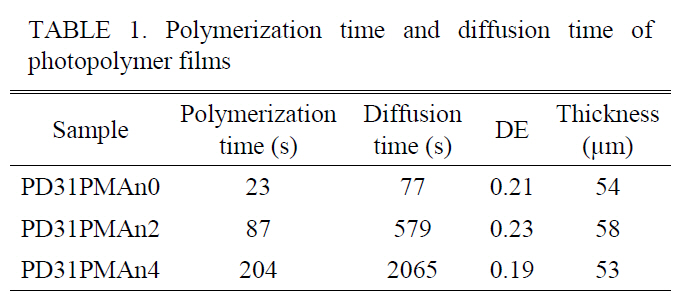
The polymerization and diffusion time were significantly longer when PMAn content was higher as summarized in Table 1. This clearly verifies that PMAn disturbs the monomer’s polymerization and diffusion, which are important for high diffraction efficiency. The optimized condition of monomer ratio between PA:DT to get high DE was found to be 3:1(PD31).
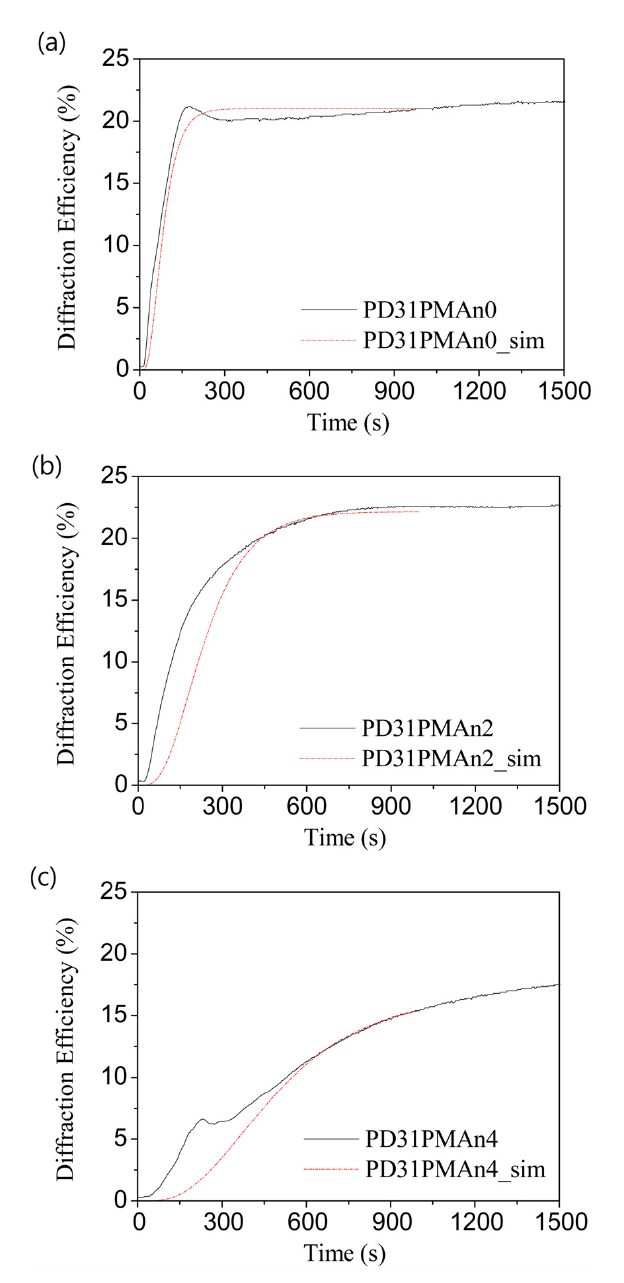
DE is calculated theoretically according to above Eq. (1)using the values of the polymerization time and diffusion time determined above in Table 1.
3.4. Fluorescence intensity changes
The fluorescence intensity after the holographic patterning
[TABLE 1.] Polymerization time and diffusion time ofphotopolymer films
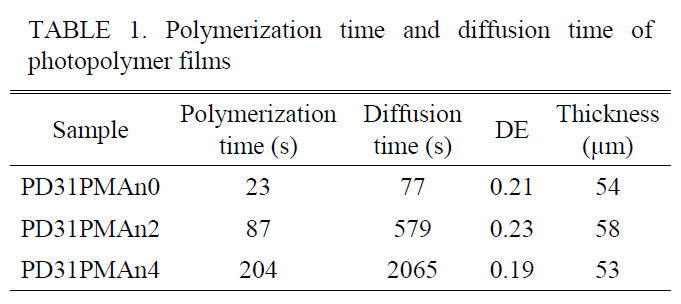
Polymerization time and diffusion time ofphotopolymer films
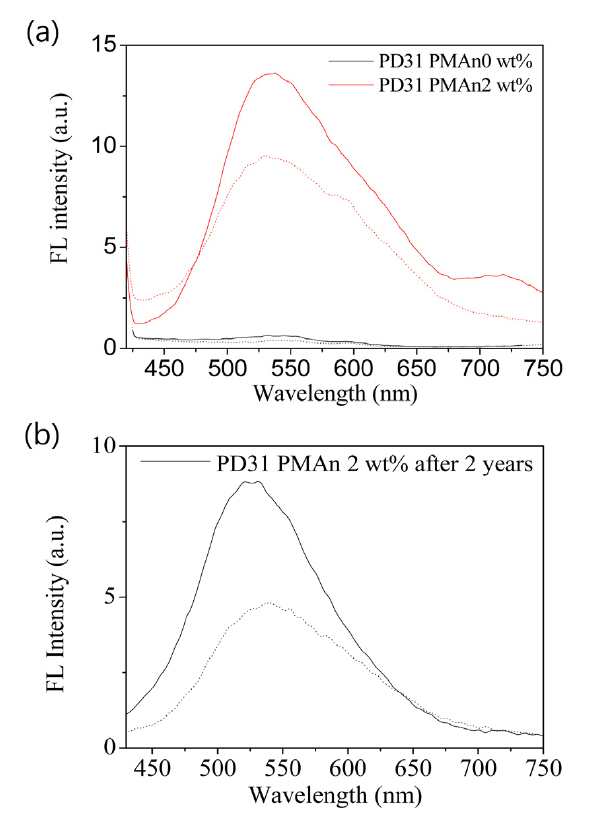
was enhanced as compared to the unpatterned sample. The fluorescent spectra of the photopolymer film before and after holographic recording are compared in Fig. 5. It showed clearly the enhancement of the fluorescence intensity in the broad emission region after patterning compared to the unpatterned sample. This may be ascribed to the micro separation of PMAn during the monomer diffusion and polymerization in the fluorescent polymer film [16]. This enhancement in FL allows further additional fluorescent patterning using a photo mask as described below. The fluorescence enhancement by holographic patterning was intact for 2 years as shown in Fig. 4 (b).
3.5. Secure fluorescent images
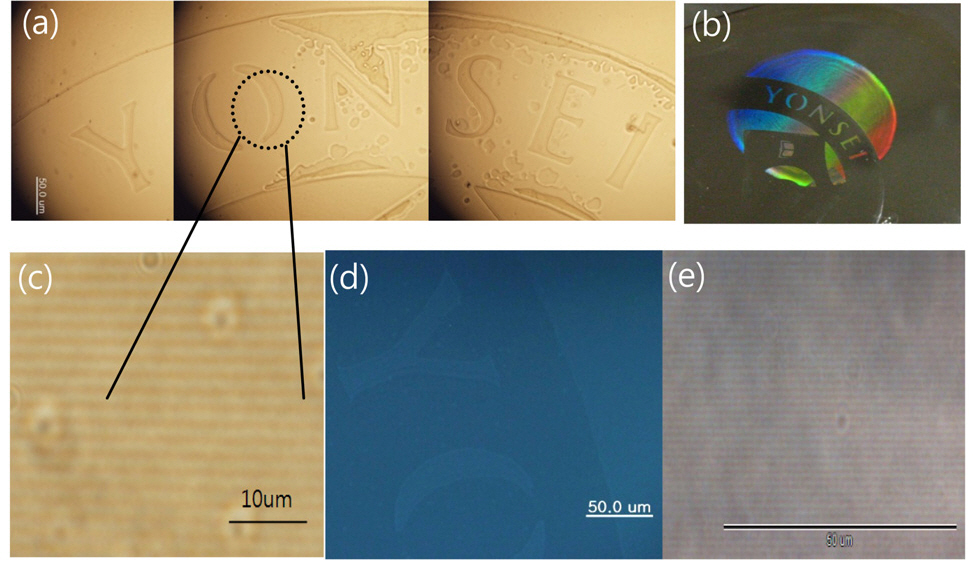
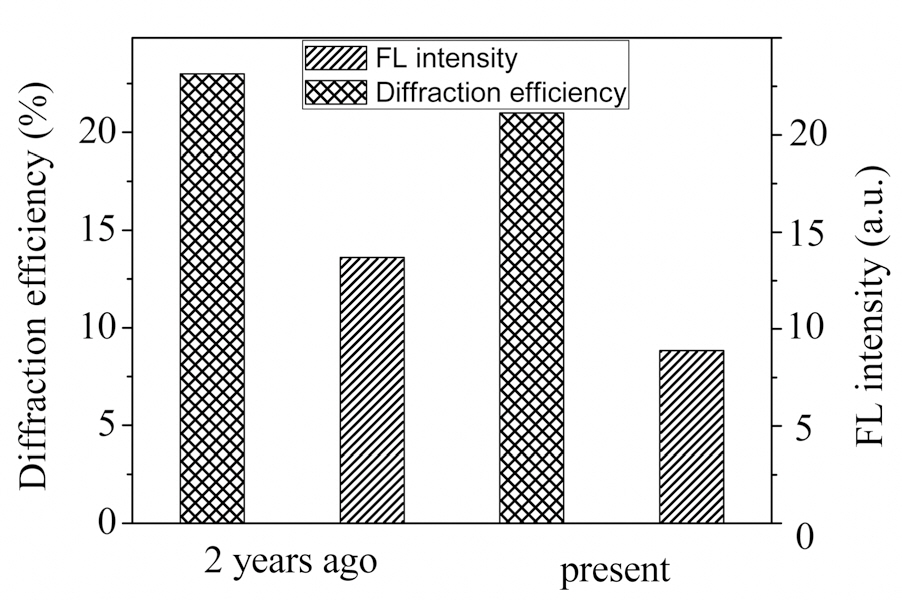
Taking advantage of the enhancement in FL by holographic patterning, we attempted to inscribe a pattern using a photomask during the holographic patterning process.Fig. 5 shows the mask pattern image as well as grating image formed by holographic patterning. The mask pattern image was well defined under an optical microscope as shown in Fig. 5 (a) and (b). The uniform gratings formed by holographic patterning were clearly observed under an optical microscope as shown in Fig. 5 (c), showing a black line of 580 nm width for the polymerized region. The mask pattern image was well defined under a fluorescence microscope as shown in Fig. 5 (d). The fluorescent intensity was increased in the recorded part. Thus this holographic patterning with a photo mask affords double pattern formation.One is photo mask patterning and the other is grating pattern by holographic recording. From this double patterning,a dual secure system is achieved. One part is changes in fluorescent intensity by photo mask recording and the other is changes in refractive index pattern made by holographic recording. This holographic recording is a result of chemical reaction. It is stable for a long time. Fig. 6 shows a comparison of FL intensity and DE for the patterned image over 2 years, ensuring long term stability of the secure image.
In this paper, the fluorescent photopolymer film containing PMAn was examined. The effects of PMAn on DE and fluorescent intensity of the photopolymer film were ascribed to the big size of fluorescent polymer, which disturbed the diffusion of monomers. Thus DE of the photopolymer containing PMAn was decreased when the concentration of PMAn was high. From photo DSC experiment, polymerization time and diffusion time were determined and it was found that the simulation result was matched to the experimental values. The enhancement of FL intensity of the photopolymer films after holographic patterning allowed double patterning with a fluorescent photopolymer film. Stable holographic pattern with a fluorescent pattern was formed by holographic recording under a photo mask. The pattern formation capability of the fluorescent photopolymer film containing PMAn can be exploited for secure films.