Cervical cancer is the second most common malignancy in women worldwide and is the only major gynecological malignancy clinically staged according to the International Federation of Obstetrics and Gynecology (FIGO) recommendations[1]. The prognostic factors identified in cervical cancer patients are tumor size, depth of stromal invasion,presence of lymphovascular tumor emboli, histologic type,and invasion of surgical margins [2, 3]. Lymph node metastasis is also closely associated with poor prognosis and decreased survival rate [4]; moreover, this metastasis is an indicator for adjuvant radiotherapy [5].
Computed tomography (CT), magnetic resonance imaging(MRI), and positron emission tomography-CT (PET-CT)have been used to detect metastatic lymph nodes in cervical cancer patients, but their accuracies were lower than expected [6, 7]. In one study of cervical cancer patients negative for metastatic lymph nodes by MRI, the sensitivity of PET in detecting metastatic lymph nodes was only 10% [8]. This low diagnostic performance was attributed to the adoption of inaccurate size criteria and a possible substantial number of small metastatic lymph nodes. Although surgical lymph node staging is an option, it is not routinely recommended because it is a specialized procedure that increases the time and cost of the surgery, and it leads to operation-related complications [9, 10]. The accurate diagnosis of metastatic lymph nodes is of clinical importance, the lack of sensitive diagnostic methods indicates the need for additional imaging modalities.
In recent years, terahertz (THz) imaging technology has shown great promise and has increasingly been performed for cancer diagnosis and treatment [11-13]. Although THz imaging may improve the diagnosis of metastatic lymph nodes in cervical cancer patients, it has not yet been evaluated in this setting. Therefore, we report a pilot study for THz imaging to detect lymph node micro-metastases in earlystage cervical cancer patients, which are usually undiagnosed by conventional imaging methods.
Two patients, aged 45 and 50 years, with FIGO stage IB1 and squamous cell carcinoma cervical cancer, respectively,underwent conventional lymphadenectomy combined with radical hysterectomy. Dissected lymph nodes were embedded in paraffin for histologic diagnosis and THz evaluation.Five paraffin-embedded metastatic lymph nodes from the two patients (three from one patient, two from the other)were sliced at 5-mm intervals in the longitudinal plane,and 4-㎛ sections were stained with hematoxylin and eosin. Because of the retrospective design of this study,our institutional review boards did not require approval or informed consent for the review of the patients’ specimens.
2.2. Image Acquisition and Measurement
All lymph node specimens were imaged using a THz time-domain spectroscopy (THz TDS) system operating in reflection mode [12]. The THz pulse was generated by an InAs wafer pumped by a Ti:Sapphire laser with a center wavelength of 790 nm, a pulse width of 100 fs, and a repetition rate of 80 MHz. The THz pulse was focused on the specimen at a normal incidence by using an off-axis parabolic mirror,and the reflected THz pulse was detected by a photoconductive antenna fabricated on a low-temperature grown GaAs. The entire THz TDS system was enclosed in a chamber and continuously purged with dry air to eliminate THz absorption by water vapor in the air. The bandwidth of the imaging setup ranged from 0.2 to 2.0 THz with a signalto-noise ratio of 2000:1. Two-dimensional THz images were obtained by raster-scanning of specimens in the x-y plane.Each scan area was ~ 20 mm × 20 mm, consisting of 40× 40 pixels with a pixel pitch of 0.5 mm, and the image quality was enhanced by using a spline interpolation.
THz images were obtained using the normalized peak-topeak amplitude of reflected THz pulses at each pixel of the specimen,
where ?is frequency,
where
Because the frequency dependence of
III. EXPERIMENTAL RESULTS AND DISCUSSION
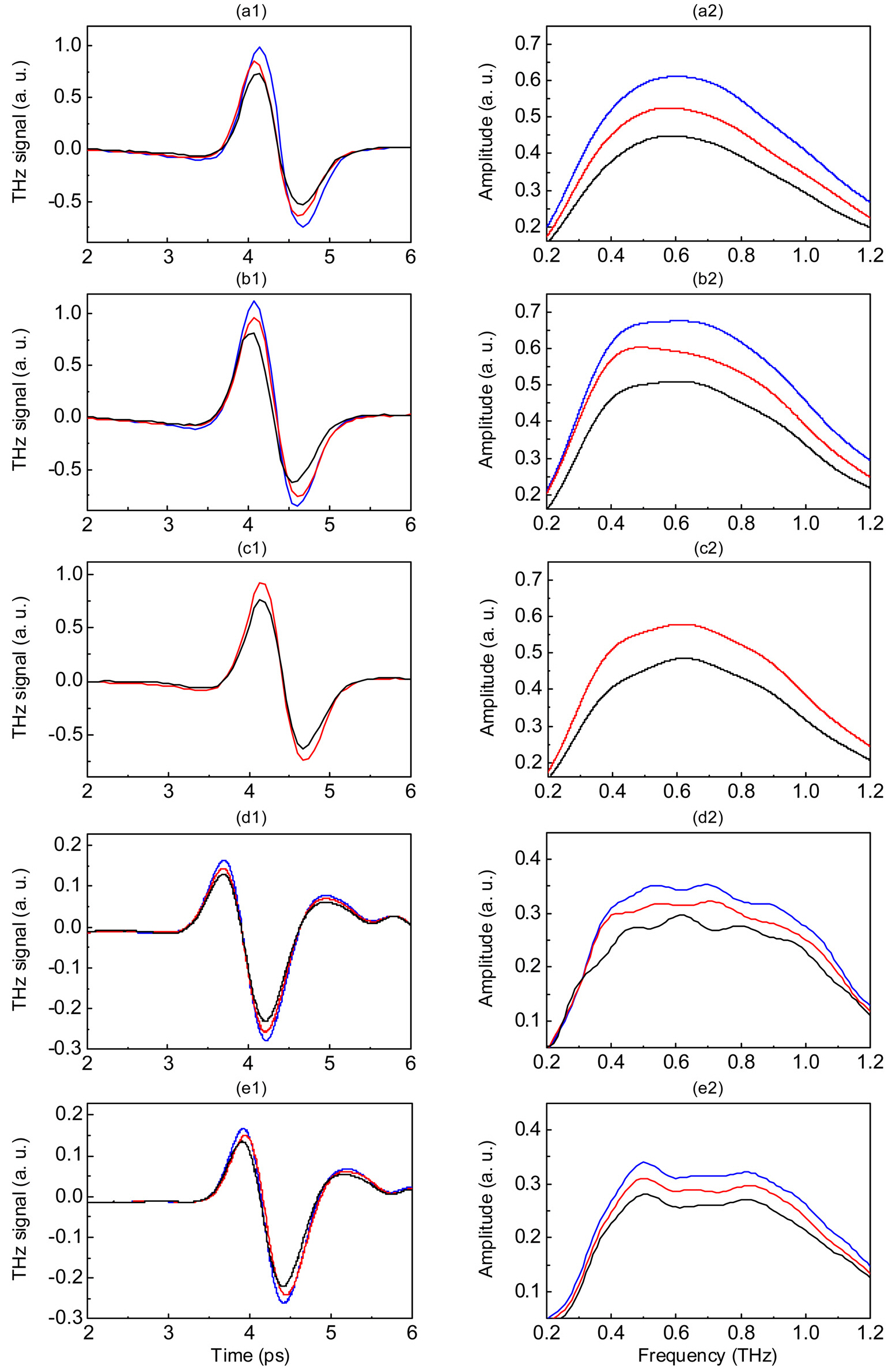
Figure 1 shows reflected THz pulses and spectral amplitudes of lymph node specimens at the selected pixels indicated in Fig. 2. For all specimens, the reflected peak amplitudes were smaller for the metastatic than for the non-metastatic portions of lymph nodes, as was the spectral amplitude in the THz frequency range. These reductions in peak amplitudes in the metastatic region were primarily due to changes in the refractive index, as shown in Fig. 2.
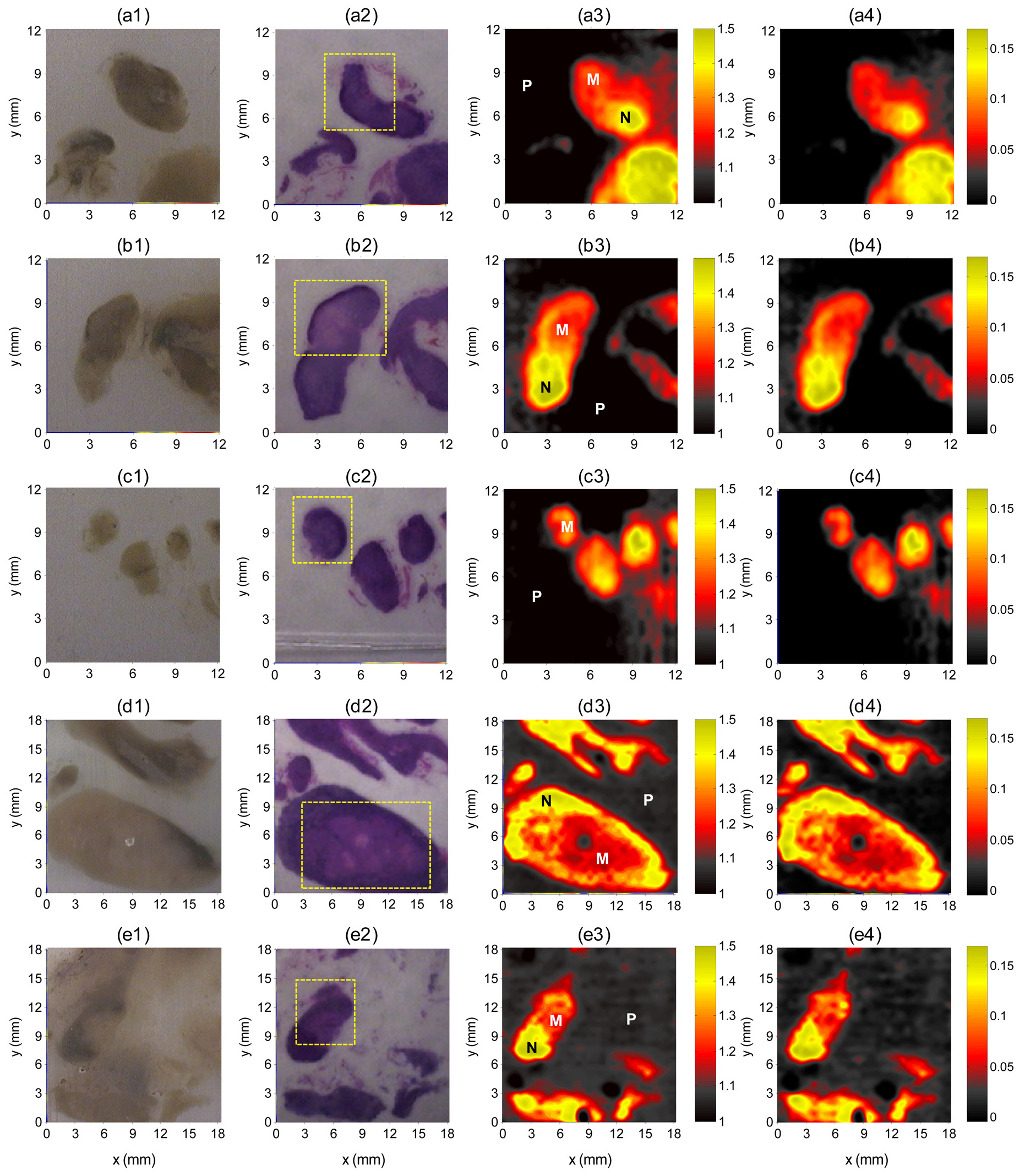
The THz images clearly differentiated metastatic regions from non-metastatic regions, even for 3-mm metastatic foci(Fig. 2) and showed good correlations with histopathologic findings. This differentiation, however, was not evident in the optical images. THz images were generated on a color scale by mapping two image parameters:
of lymph nodes was 4.7 ± 0.5%.
Lymph node metastasis is an important factor influencing prognosis and therapeutic strategy in patients with cervical cancer. Although CT and MRI have been used to detect metastatic lymph nodes, including measurements of size,shape, presence of necrosis, and enhancement characteristics,these methods cannot efficiently differentiate malignant from hyperplastic lymph nodes [15-17]. Many of these metastatic
lymph nodes are smaller than 1 cm, the usual diagnostic size criteria for metastatic lymph nodes, thereby reducing the diagnostic accuracy of CT and MRI [18]. Although PET scanning diagnoses metastatic lymph nodes by assessing the abnormal accumulation of FDG, PET is expensive and less widely available, and has lower spatial resolution. The pooled sensitivities of CT, MRI, and PET were found to be only 52%, 38%, and 54%, respectively, in region- or nodebased comparisons, which are too low to replace surgical staging [19, 20]. Thus, there are as-of-yet unmet clinical needs for the accurate imaging diagnosis of metastatic lymph nodes and detection of small metastatic nodes, i.e., diagnoses that better reflect the actual histological processes.
Our pilot study showed that THz images delineated metastatic foci in lymph nodes to an extent similar to those of histopathologic methods. Although the overall correlation of metastatic foci and THz images were good, peak reflection amplitudes at the edges of the lymph nodes were lower because of averaging with the adjacent paraffin, which has much lower reflected peak amplitudes. This edge effect may be responsible for the false positive findings in benign portions of the lymph nodes. We found that the smallest metastatic focus delineated on THz imaging was approximately 3 mm in its largest dimension. Although these were specimen images,we believe that our findings are meaningful as a pilot study for future investigations of this imaging modality in the diagnosis of micro-metastatic lymph nodes, which are usually undiagnosed by conventional imaging methods.
Earlier studies have suggested that water content is a major contributory factor for contrast in THz images since tumors generally contain a higher water content than normal tissue and liquid water has high attenuation for THz waves[21-23]. Although several other studies have used paraffinembedded tissues [24-26], the fundamental mechanisms underlying the contrast in THz images of the dehydrated paraffin-embedded samples that differentiate tumor from normal tissue are not clear at this stage. However, the different contrasts for tumors and normal tissues in THz images of dehydrated paraffin-embedded samples may be due to other components, including cell density, proteins, and DNA.Thus, paraffin-embedded tissue samples may be useful for assessing cancer biology using THz imaging and for investigating THz contrast mechanisms, other than the effects of water.
We have shown here that THz imaging can clearly delineate the metastatic portions of the lymph nodes as small as 3 mm. Two THz images by using peak-to-peak amplitudes and refractive index changes were almost identical, demonstrating that THz images can also determine refractive indices of lymph node specimens. THz imaging is a promising technique for the detection of micro-metastatic lymph nodes that cannot be detected by conventional imaging methods,but technical improvements are needed prior to its clinical application.