The brown alga Rosenvingea and various other genera are placed in the family Scytosiphonaceae (order Ectocarpales) based on molecular evidence (Peters and Ramirez 2001) and several morphological characters:erect macroscopic parenchymatous thalli with an apical to subapical meristem, vegetative cells with a single chloroplast and prominent pyrenoid, medulla of large, vacuolate,colourless cells and cortex with small dark brown cells containing physodes, phaeophycean hairs scattered on thallus surface, ascocysts (clavate colorless cells with numerous physodes) in plurangial sori present in some genera and plurangial reproductive structures in scattered, round to linear sori. Most genera (e.g., Scytosiphon, Colpomenia, Myelophycus, Hydroclathrus, Iyengaria, Petalonia) are unbranched cylinders, flat blades or cushions whereas Rosenvingea and Chnoospora are branched cylinders or narrow blades.
Seven species of Rosenvingea are currently accepted with the type species being R. sanctae-crucis Børgesen (Børgesen 1914) known originally from the Caribbean and now reported as pantropical (Guiry and Guiry 2010). Rosenvingea intricata (J. Agardh) Børgesen is also pantropical, forming dense mats of flattened, often co-adhering, fronds 2-8 mm wide and up to 15 cm long (Jaasund 1976, Huisman 2000, Abbott and Huisman 2004). Rosenvingea nhatrangensis Dawson has branches that are wide (10-15 mm) and flat. It is known only from southeast Asia and Western Australia (Dawson 1954, Huisman and Borowitzka 2003). Rosenvingea floridana (W. R. Taylor) W. R. Taylor has narrow (< 2 mm) branches and is known from the Caribbean (Taylor 1960), Pacific Mexico (Norris 2010), California (Abbott and Hollenberg 1976) and southwest Asia (Guiry and Guiry 2010). It is similar to Rosenvingea antillarum (P. Crouan et H. Crouan) M. J. Wynne that is slightly narrower (1 mm) and reported from the Atlantic region (Caribbean, Canary Islands, Angola) (Guiry and Guiry 2010). Rosenvingea fastigiata (Zanardini) Børgesen is found in the Asia-Pacific (Guiry and Guiry 2010) and has terete (< 3 mm) dichotomous fronds that form either matted clumps or individual thalli (Littler and Littler 2003).
Rosenvingea orientalis (J. Agardh) Børgesen also is pantropical with long (to 30 cm) and narrow (< 2 mm) tubular to compressed fronds that are dichotomously branched in the upper thallus (Jaasund 1976, Kraft 2009). All Rosenvingea species have a hollow medulla, in contrast to Chnoospora, considered the most closely related genus, that is dichotomously branched with a solid medulla (Abbott and Huisman 2004). The reproductive patterns are largely unknown for Rosenvingea. Plurilocular sporangia (plurangia) are known in all species but unangia are unreported. The characters distinguishing the species show some overlap and the lack of culture and life history investigations create further problems.
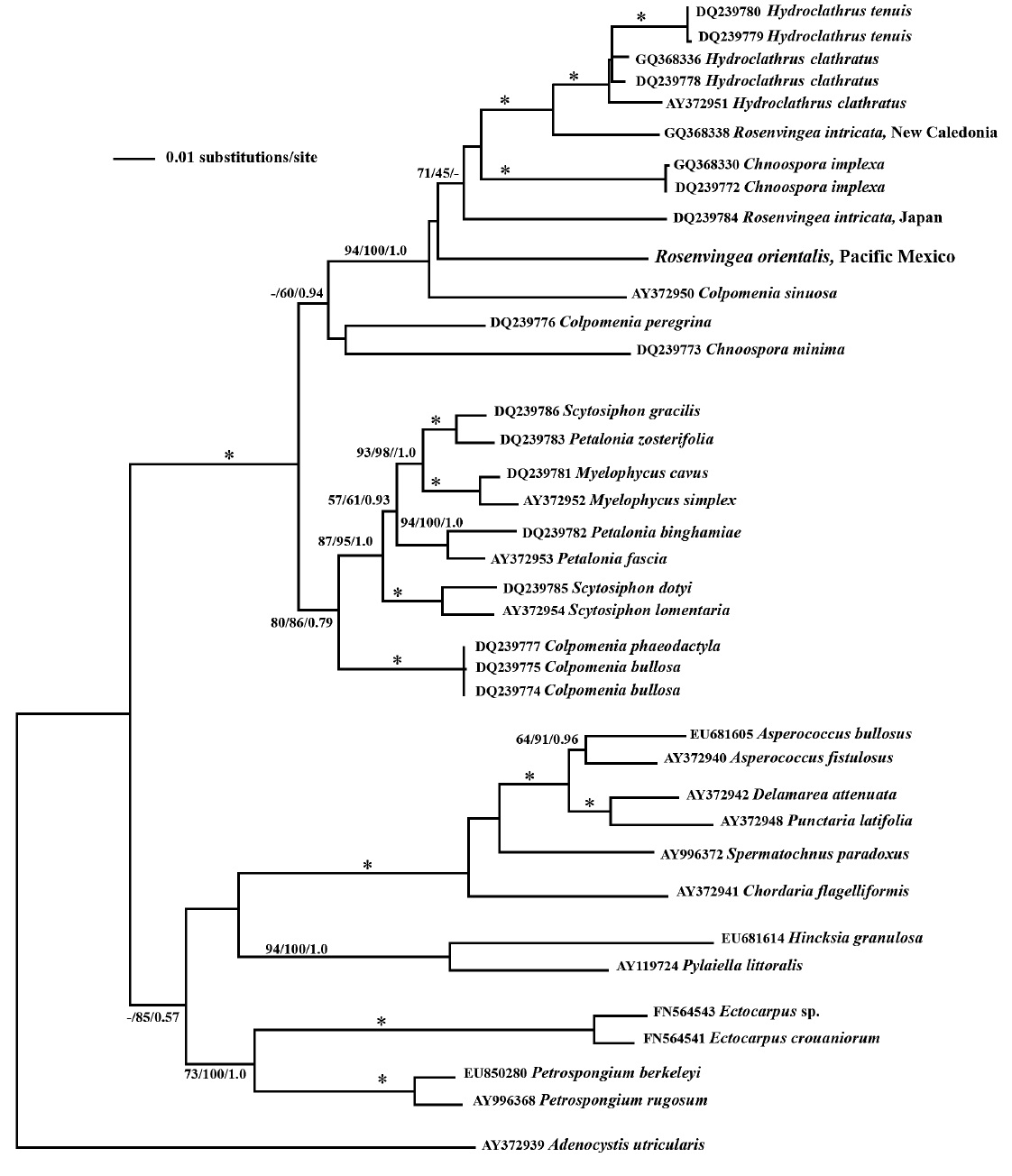
Molecular data presented by Cho et al. (2006), based on plastid-encoded psaA and rbcL sequence data, clearly indicate that the family Scytosiphonaceae needs considerable reorganization at the generic level as several genera (e.g., Colpomenia, Chnoospora, Scytosiphon) are not monophyletic. Their study only used one Rosenvingea isolate, R. intricata from Japan. Another study of the higher level taxonomy of the Phaeophyceae by Silberfeld et al. (2010), used an isolate of R. intricata from New Caledonia.
In Pacific Mexico R. sanctae-crucis, R. antillarum, R. floridana and R. intricata are recorded (Pedroche et al. 2008, Norris 2010). Our collections from Chiapas, Mexico revealed a new specimen that was identified morphologically as R. orientalis, the first record of this species from Pacific Mexico. Its availability in culture provides an opportunity to understand its reproduction and morphology more completely and to compare it genetically to other species of the genus.
Rosenvingea orientalis was obtained in a mixed culture of Bostrychia radicans (Montagne) Montagne, Caloglossa leprieurii (Montagne) G. Martins and Stylonema alsidii (Zanardinii) K. M. Drew collected from Boca del Cielo, Chiapas, Mexico (15º51.171′ N, 93º40.229′ W) on 11 March 2009. The culture (JAW 4775) has been given to the Kobe University Marine Algal Culture Collection.
General culture methods are provided in West and Zuccarello (1999). The initial culture was established in a 50 × 70 mm crystallizing dish with 70 mL of 30-32‰ seawater, quarter-strength Modified Provasoli’s Medium (MPM) (West 2005) at 18-22°C, 2-3 ㎛ol photons m-2 s-1 cool white light emitting diodes, 12 : 12 light-dark diurnal cycle. For faster growth 30-35 ㎛ol photons m-2 s-1, a rotary shaker at about 70 rpm and full strength MPM were used. For slower growing low-light cultures the medium was changed every 3 wks. With faster growing cultures in 500 mL deep storage dishes the medium was changed weekly. To follow spore germination a 22 mm cover slip was placed in each 50 × 70 mm crystallizing dish with 70 mL of MPM and a 0.1 mL of spore suspension was added. Photos were taken every 3-4 days. Cell numbers of the basal system were counted and overall dimensions measured for 10-20 sporelings each time. Also the length and diameter of developing erect shoots were measured.
Most photography was done on live unfixed specimens using bright field optics on a Zeiss GFL microscope (Carl Zeiss, Thornwood, NY, USA) with a Canon G3 camera (Canon, Tokyo, Japan) and Photoshop CS (Adobe Systems Incorporated, San Jose, CA, USA) to capture the image. To more clearly see the arrangement of cells in mature shoot tips, a blade segment (2 mm long) was microwaved in distilled water for 20 s to shrink cells at the apex, mounted on a slide with a cover slip and then gently pressed to flatten and spread the tissue.
Total DNA was isolated from dried samples using a modified CTAB procedure (Zuccarello and Lokhorst 2005). The molecular marker used, following previous work on the family by Cho et al. (2006) was the plastid-encoded psaA gene. The gene was amplified in two overlapping sections with the primer combination psaA130F-psaA940R and psaA870F-psaA1760R from Yoon et al. (2002). Polymerase chain reaction (PCR) conditions consisting of an initial denaturation of 5 min at 94°C, followed by 37 cycles of 1 min at 94°C, 1 min at 45°C and 1 min at 72°C, with a final 5 min extension at 72°C.
PCR products were cleaned using ExoSAP-IT (USB Corp., Cleveland, OH, USA) and commercially sequenced (Macrogen, Inc., Seoul, Korea). Available sequence data of the Scytosiphonaceae for psaA were obtained from GenBank mostly from Cho et al. (2006). We used Adenocystis utricularis as the outgroup following Cho et al. (2006)
Maximum-parsimony trees were constructed in PAUP* (Sinauer Associates, Inc., Sunderland, MA, USA), using the heuristic search option, 500 random sequence additions, tree-bisection-reconstruction branch swapping, unordered and unweighted characters, gaps treated as missing data and 1 million rearrangements per replicate. Support for individual internal branches was determined by bootstrap analysis (Felsenstein 1985), as implemented in PAUP*. For bootstrap analysis, 1,000 bootstrap data sets were generated from resampled data (10 random sequence additions).
Likelihood settings were calculated with Modeltest version 3.7 (Posada and Crandall 1998). The best fit model selected by Akaike information criterion. When
the sequence evolution model had been determined, maximum-likelihood was performed in PAUP* using the estimated parameters (substitution model, gamma distribution, proportion of invariable sites, transition?transversion ratio and 5 random additions). Bootstrap values using maximum likelihood analyses were performed on PHYML, with 100 replicates and the same model parameters.
Bayesian analyses were performed using MrBayes version 3.0b4 (Huelsenbeck and Ronquist 2001) with a random starting tree and run for 3 × 106 generations in each of the two four-chain runs, keeping one tree every 100 generations. The first 0.5 × 106 generations (burn-in) were discarded and the remaining 25,000 trees (representing 2.5 × 106 generations) were used to calculate a posterior probability tree topology.
In culture fully developed fronds reached 6 cm in length and a width up to 2 mm with 4-5 orders of dichotomous branching occurring primarily in the upper half of the developing shoots. Some adventitious secondary branching developed at irregular intervals along the lower shoot. From the small basal mat many secondary shoots arose (Fig. 1A). Based on these morphological features it seems closest to R. orientalis than the other species presently accepted. A key to the species is seen in Table 1.
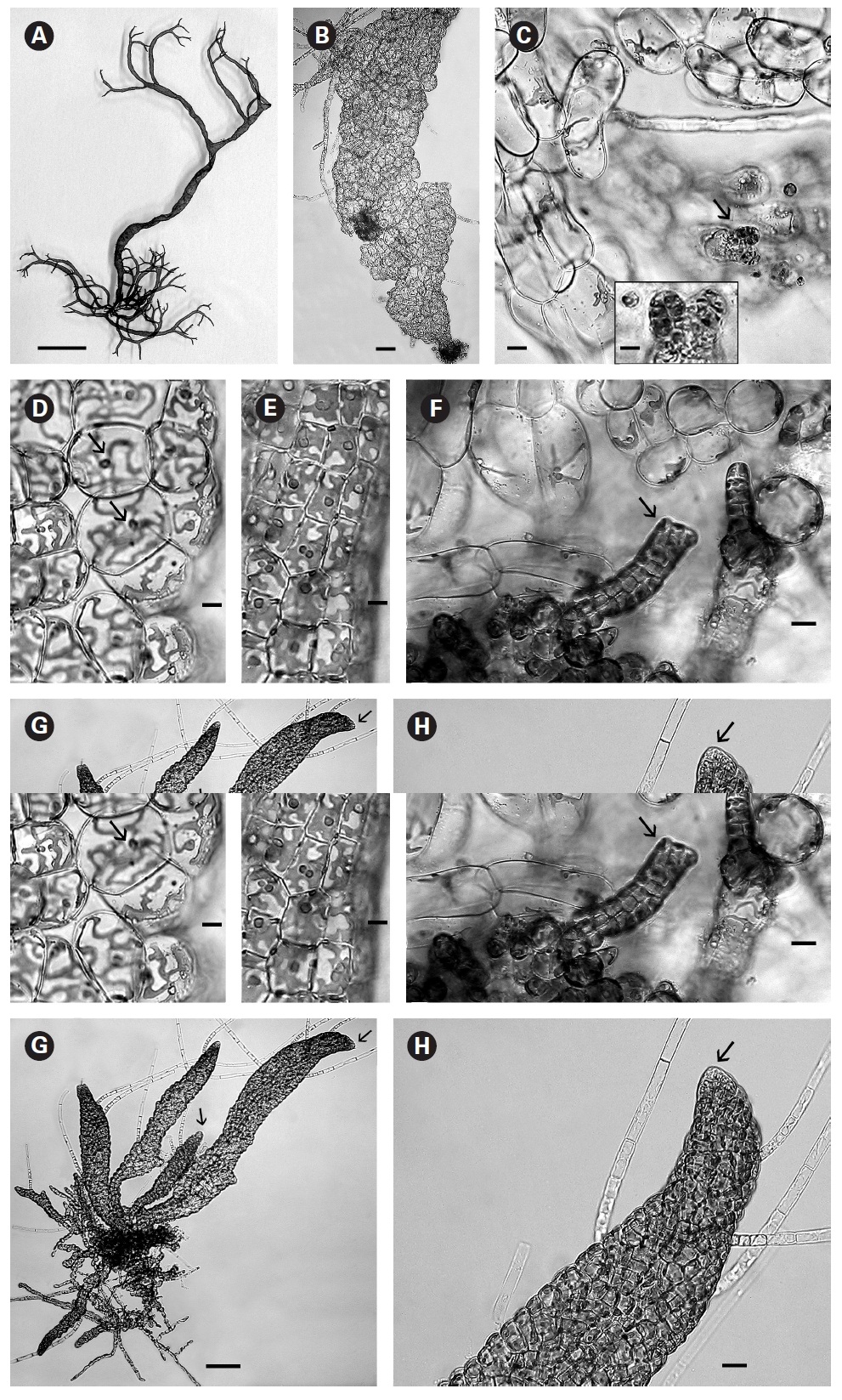
When first observed in rough culture R. orientalis was epiphytic on Bostrychia radicans. A single clean filament was isolated into low nutrient medium and low light. Growth was very slow initially and after 3-4 weeks the filaments formed loosely organized multiaxial shoots comprised of large conspicuous cells with a very large central vacuole (Fig. 1B). On these large inflated cells of openly branched filaments tiny plurangial ‘packets’ developed (Fig. 1C and inset). Each vegetative cell contained a single, long, thin, multilobed parietal chloroplast with one pyrenoid (Fig. 1D).
In brighter light (> 20 ㎛ol photons m-2 s-1) smaller rectangular cells developed with larger chloroplasts in the cortex. In some cells paired pyrenoids possibly indicated the onset of cell division (Fig. 1E). A few small (< 1 ㎛ diameter) colourless bodies, presumed to be physodes, similar to those observed by Feldmann (1949) in R. intricata, were visible in the cytoplasm. These physodes were most numerous in the cortical cells of larger thalli grown in bright light on the shaker.
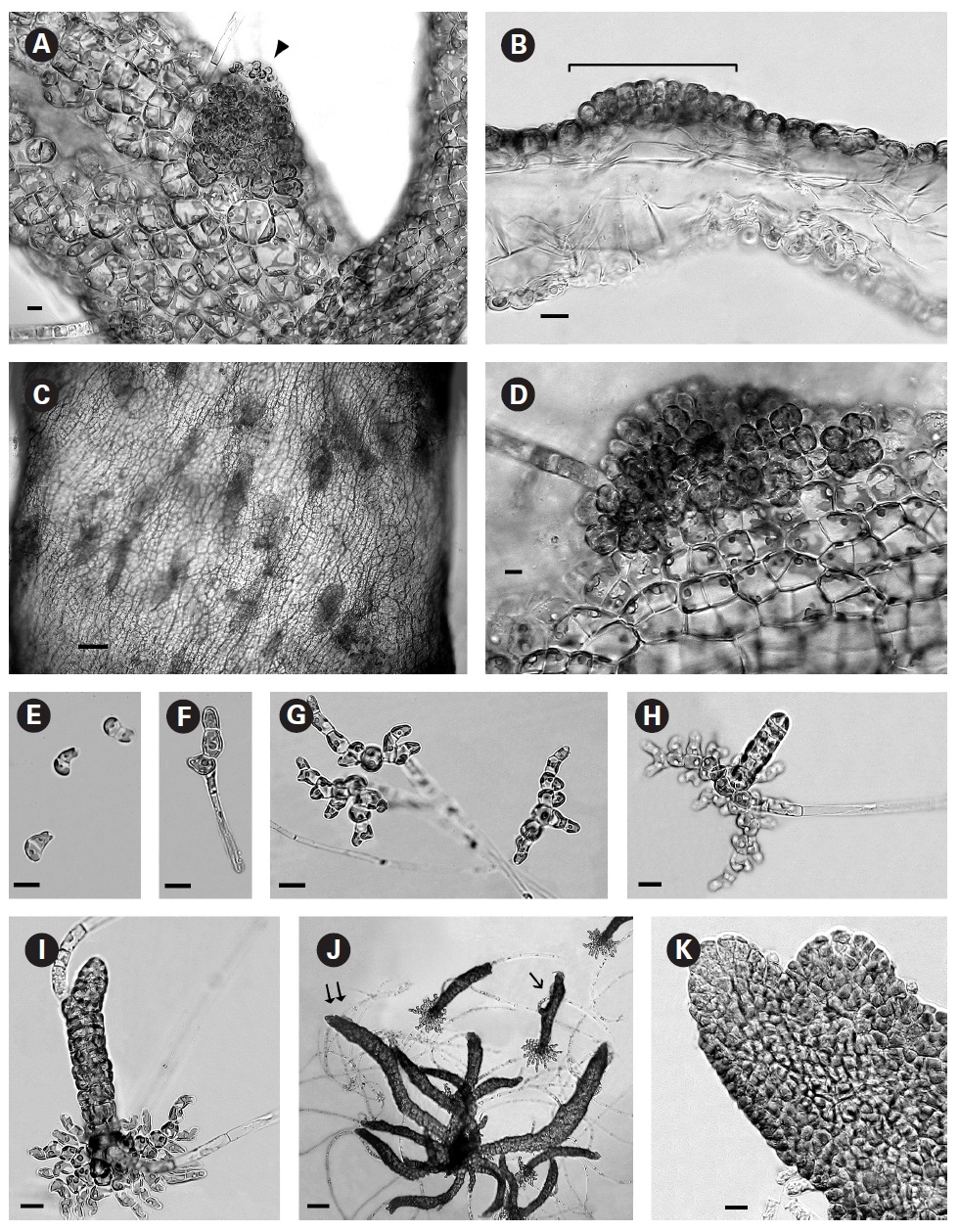
When thalli with loosely coherent filaments were transferred from low light to brighter light on the shaker numerous, compact dark brown shoots developed (Fig. 1F). After 15 days the shoots reached 300 ㎛ long. At 25 days they became about 800 ㎛ long and 100 ㎛ wide with a well-defined medulla and cortex and phaeophycean hairs scattered along the axis (Fig. 1G). The older and longer shoots shifted from a cylindrical form to slightly compressed fronds and all had a hollow center enclosed by the large colourless medullary cells. The apex had a single apical cell and compact cortex with numerous phaeophycean hairs (Fig. 1H). In larger thalli with a compact cortex small, round plurangial sori developed in the mid-regions (Fig. 2A). In cross section, older wider shoots (1.2 mm) were about 120-140 ㎛ thick, with a medulla of large colorless cells and a cortex of a single cell layer (Fig. 2B). Also visible were plurangial sori about 10 cells or more wide (Fig. 2B). Sectioning caused partial collapse of the medulla so the hollow center was not evident. No hyphae were seen in the medulla of longitudinal sections, near the base of older fronds, as was described by Børgesen (1914) for R. sanctae-crucis.
In older compressed fronds the elongate and mature linear sori followed the slightly oblique pattern of cortical cells along the axis and were generally 100-300 ㎛ long and 60-100 ㎛ wide (Fig. 2C). The plurangial sori were usually two cells deep and in surface view each plurangium appeared to be composed of four cells quadrate to rectangular in shape and somewhat variable in size, 8-15 ㎛ wide and 12-20 ㎛ long (Fig. 2D). Visible within each sorus were several colorless phaeophycean hairs about 5-10 ㎛ wide at the base (Fig. 2D) and up to 1 mm long, before abscising at one or more points along the length.
The zoospores discharged readily and were about 6-8 ㎛ long with two heterokont flagella, a single peripheral chloroplast bearing a pyrenoid and lateral anterior eyespot. In bright light the spores settled on the glass and germinated rapidly. At day 1 (20 hours old) most sporelings were 2 cells long (8-19 ㎛) (Fig. 2E) and did not show evacuation of the original spore that is seen in germination of other Scytosiphonaceae (e.g., Petalonia and Scytosiphon) (Wynne 1969, Hori 1993, Kogame 1998). At day 3 many were 3 cells long (35-40 ㎛) with a single hair (Fig. 2F). By day 7 each sporeling had 8-13 cells (65-70 ㎛ long) and was branched 3-5 times (Fig. 2G). Only a single hair was still evident in each sporeling. The sporelings were all prostrate and attached to the glass. On day 9 the basal systems were 100-130 ㎛ overall with a total of 30-40 cells and many branches and still bearing only a single hair. Nearly all sporelings had developed a single uniseriate erect shoot 25-60 ㎛ long (Fig. 2H). By day 12 erect shoots were 100-150 ㎛ long and had developed a single subapical hair still retaining the single-cell apex (Fig. 2I) The basal discs were about the same overall dimensions (115-120 ㎛) as for day 9 but the number of cells increased to 39-45 while still retaining a single hair. Basal cell divisions were primarily at the apices of the marginal branches but some intercalary cells also formed laterals and the cells of the whole base were becoming cohesive (Fig. 2I).
The shoots of clumped plants appeared to grow faster than solitary ones, measuring 0.5 to 1.2 mm in length. Phototropic growth of the erect shoots of solitary fronds was evident throughout development (Fig. 2J). At some late stage in development shoots appeared to switch from single celled to multi-celled apices (Fig. 2K) but it was difficult to determine when. Cell divisions were primarily in the apical to subapical regions.
Our psaA phylogeny is seen in Fig. 3. The phylogenetic relationships showed a well-supported Scytosiphonaceae (fully supported by bootstrap and posterior probabilities) consisting of the genera Chnoospora, Colpomenia, Hydroclathrus, Myelophycus, Petalonia, Rosenvingea and Scytosiphon. While the family is well supported the monophyly of many of the genera is not. These results are similar to those from Cho et al. (2006). Our results add another member of the Scytosiphonaceae, Rosenvingea orientalis, to the phylogeny. Maybe unsurprisingly, the
phylogenetic placement of our sample does not group with other samples of Rosenvingea. While R. orientalis is sister to R. intricata from Japan it does not group with R. intricata from New Caledonia.
Our combined morphological, life cycle and molecular phylogeny studies suggest that the plant collected in Mexico is R. orientalis, previously unreported from this area,. This is also the first study to show the life cycle in culture of a Rosenvingea species. It evidently lacks a sexual life cycle reproducing asexually through plurangia.
Reproductive patterns in various genera of the Scytosiphonaceae are variable in different taxa and different regions. Scytosiphon species have been extensively investigated revealing sexual life histories with crusts bearing meiotic unangia and upright fronds with plurilocular gametangia (e.g., Hori 1993, Kogame 1998). Colpomenia sinuosa (Mertens ex Roth) Derbes et Solier in Castagne and Hydroclathrus clathratus (C. Agardh) Howe (Kogame 1997) and Chnoospora implexa J. Agardh (Kogame 2001) have similar heteromorphic sexual life histories.
Only plurangia occurred in our cultured isolate of R. orientalis and only plurangia are recorded by numerous authors (e.g., Feldmann 1949, Taylor 1960, Jaasund 1976, Huisman 2000, Abbott and Huisman 2004, Kraft 2009) in field specimens of various Rosenvingea species indicating that the life history is probably asexual. It remains to be determined if other investigators undertake further culture studies with different isolates grown in short day-long day conditions and high and low temperatures whether or not a heteromorphic sexual life history might be revealed.
Ascocysts were not observed in R. orientalis nor have they been recorded in the other Rosenvingea species although they are evident in some other members of the Scytosiphonaceae, e.g., Scytosiphon lomentaria (Lyngbye) Link and Scytosiphon tenellus Kogame (Kogame 1998), Colpomenia peregrina Sauvageau and Colpomenia phaeodactyla M. Wynne et J. Norris from Korea (Boo unpublished). Kogame (1998) considered ascocysts to be an important taxonomic character.
The type species R. sanctae-crucis was described by Børgesen (1914, Fig. 15d) as having hyphae in the hollow medulla at the basal part of the frond. However internal hyphae have not been reported by any subsequent authors and was not observed in this isolate of R. orientalis. That may be a critical feature only at the species level but definitely requires confirmation.
A key (Table 1) was constructed to aid in the identification of the seven Rosenvingea species currently recognized. Rosenvingea nhatrangensis is the most distinctive because of its greater branch width of 10-15 mm and distinct plurangial sori having a central depression with a cluster of phaeophycean hairs. Rosenvingea fastigiata is up to 3 mm wide with dichotomous branching. Rosenvingea intricata forms compact mats with branches often adhering together. Rosenvingea sanctae-crucis, R. antillarum, R. floridana and R. orientalis all have narrower branches (< 2 mm) and varied branching patterns, irregular, opposite and dichotomous.
The phylogenetic relationships seen in this study, based on psaA gene sequence, are similar to those of Cho et al. (2006) and suggest that: 1) the characters used to identify genera have arisen multiple times, for example the blade morphology of Petalonia plus the hollow branch morphology of Rosenvingea; and 2) that serious taxonomic work needs to be done in this group to find characters that reflect its evolution as seen in gene phylogeny.