Silica-scaled chrysophytes (Synurophyceae) are important components of the species composition and biomass in freshwater environment worldwide, and the planktonic flagellate species Mallomonas elongata Reverdin is one of the most common and widespread synurophytes (Siver 1995, Kristiansen 2005). Some species of this algal group including M. elongata often form very dense blooms (Hoffmann and Wille 1992, Kim and Hwang 2001, Lee et al. 2005), which negatively affect the taste and odor of potable water (Nicholls 1995). Although the temperature, pH, conductivity and nutrients (nitrogen and phosphorus) are primary factors controlling the distribution and seasonal occurrence of silica-scaled chrysophytes (Siver 1995), few comprehensive studies have been conducted on the growth characteristics of individual taxa. An important aspect to manage bloom formation in any group of organisms is an understanding of the relative importance of each environmental factor in controlling the occurrence and distribution of the group in the field. Furthermore, understanding the ecophysiological growth characteristics of individual species through laboratory culture experiments is necessary to control and manage blooms. The autecological characteristics of specific species through culture experiments can show the influence of variable parameters such as temperature, pH, nutrients (N, P, and Si), and light on their growth and development. However, only a few studies on the growth characteristics of silica-scaled chrysophytes through in vitro laboratory culture experiments have been conducted (Healey 1983, Wee et al. 1991, Saxby-Rouen et al. 1997, Lee et al. 2007, Kim et al. 2008).
A dense bloom of M. elongata was observed during early spring in a small shallow eutrophic pond, located at the campus of Kyungpook National University, South Korea. Laboratory experiments using unialgal cultures of M. elongata isolated from the pond were performed to investigate the growth characteristics of this flagellate species at varying concentrations of Si and light intensities. A comparison of the natural and laboratory nutrient conditions required for optimal growth of this species is presented, and factors that may have caused discrepancies between the natural and laboratory conditions are discussed.
Jido pond is located at Kyungpook National University, South Korea (128°37| E, 35°53| N). It has a surface area of approximately 200 m2 and a mean depth of 0.7 m. M. elongata was isolated by pipetting single cells from Jido pond water samples collected with a plankton net (mesh size 40 ㎛) on May 5, 2005. Unialgal stock cultures were maintained in DY III medium buffered to pH 7.0 with 2-(N-morpholino) ethanesulphonic acid (MES), at a temperature of 15 ± 1°C and a light intensity of approximately 80 ㎛ol m?2 s?1 (cool white fluorescent light) on a 16 : 8 h light : dark cycle.
For the Si experiments, clones selected from stock cultures in exponential growth were first adapted to Si-limited media for 1 week, and then inoculated into fresh media at an initial cell density of approximately 800 cells mL?1. Growth experiments at various Si concentrations were conducted in triplicate in 125 mL Erlenmeyer flasks under continuous white fluorescent illumination of approximately 100 ㎛ol m?2 s?1 intensity at 15°C and pH 6, which was the optimal temperature and pH condition for growth in a previous study (Lee et al. 2005). The effect of light intensity on growth was examined at pH 6 and two temperature conditions (15°C and 18°C) to verify the results of our previous studies that showed a different effect based on strains among the same species (Lee et al. 2005, 2007). The light was measured with a TES 1332A digital lux meter (TES Electrical Electronic Corp., Taipei, Taiwan), and light values were converted to photon flux density (㎛ol m?2 s?1). The number of cells was enumerated at 2- or 3-day intervals, and counts were continued for 15 days until the stationary or decline phase was reached. Samples were fixed in Lugol’s solution, and cell numbers were determined using a Sedgwick-Rafter chamber (PhycoTech Inc., St. Joseph, MI, USA).
Growth rates were expressed as μ = (ln N2 ? ln N1) / (t2 ? t1), where N2 and N1 are the number of cells during the period of exponential growth at times t2 and t1, respectively. Specific growth rates (μ) were calculated during the exponential growth periods between day 0 and days 6 74or 9 for each species. The pH of the medium was adjusted by adding of HCl or NaOH. Strains are available from the culture collection of the authors.
Details regarding environmental factors at the study site are described in a previous study (Lee et al. 2005). The pond water was highly eutrophic, and total nitrogen and phosphorus concentrations ranged from 3.714 to 8.702 mg L?1 and from 0.026 to 0.049 mg L?1, respectively (Lee et al. 2005).
The dominant species in the planktonic bloom were identified by electron microscopy as M. elongata and Synura petersenii.The seasonal patterns of total phytoplankton and the two synurophytes have been described by Lee et al. (2005) and Kim et al. (2008), respectively. The M. elongata population appeared in early spring, maintained a high density for a short period (March 21 to April 7), and accounted for 27.6-65.5% of the total phytoplankton population. At peak population abundance, the density of M. elongata was 17,600 cells mL?1, but gradually decreased as water temperature increased in April.
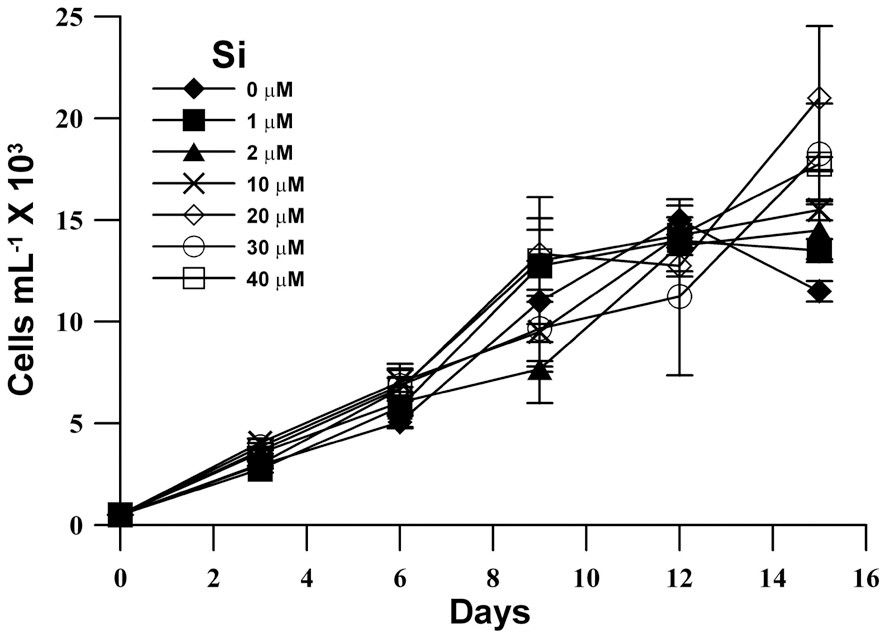
The population growth in unialgal cultures of M. elongata was examined at various Si concentrations (Fig. 1). Although M. elongata exhibited a maximum population growth at an Si concentration of 20 ㎛ol, maximum
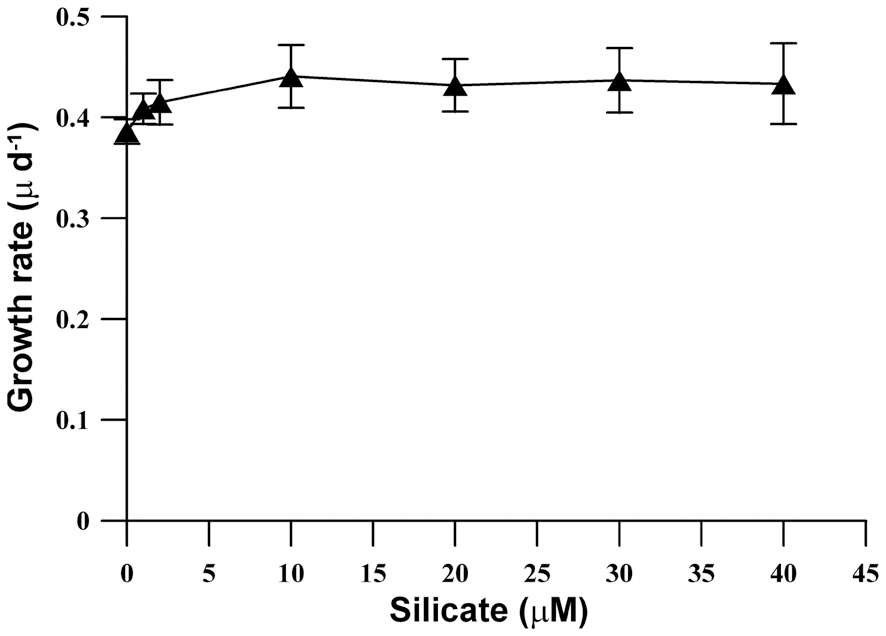
population density was similarly reached at all Si concentrations, and a high population density (15,000 cell L-1) was also observed in Si-limited medium. Furthermore, the growth rates of M. elongata increased gradually kras the Si concentration was increased to 10 ㎛ol, but the higher concentration had no effect (Fig. 2).
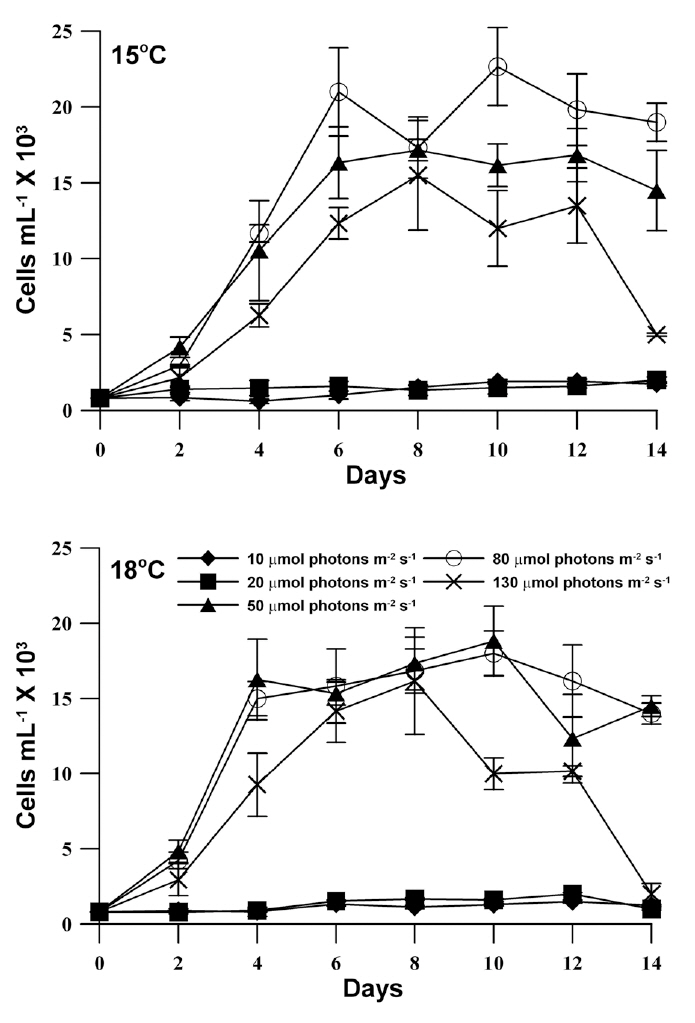
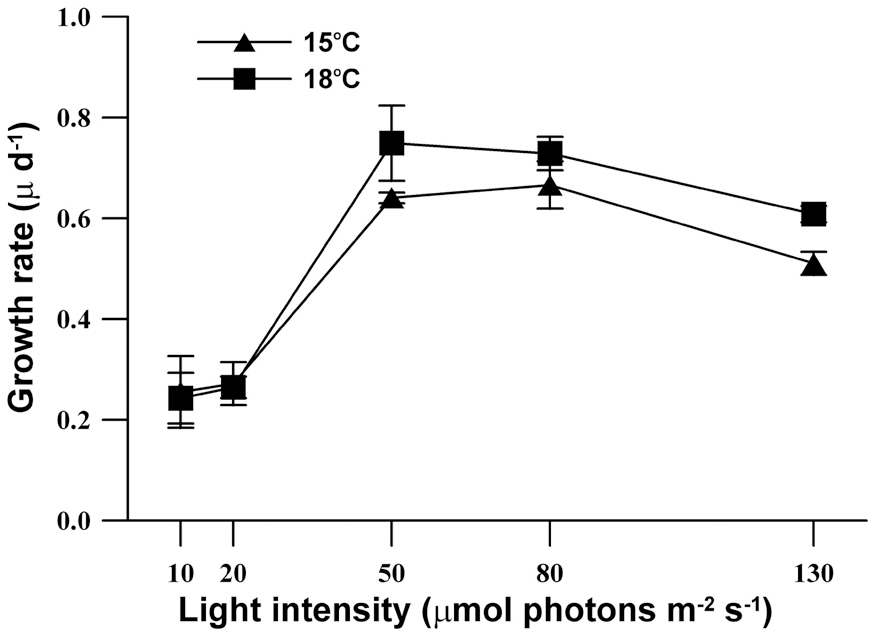
The growth response of M. elongata exposed to variable light intensities at 15°C and 18°C is shown in Fig. 3. In general, population growth patterns at both experiment temperature conditions similarly exhibited within the range of experimental light conditions (light intensities of 10-130 ㎛ol m-2 s-1). Under both experimental temperature conditions, M. elongata exhibited growth at all experimental light intensities, but very low growth appeared below a light intensity of 20 ㎛ol m-2 s-1. Moreover, population growth increased as light intensity was increased up to an intensity of 80 ㎛ol m-2 s-1. At a light intensity of 130 ㎛ol m-2 s-1, population growth gradually increased, maximum density was reached at 8 days, and then rapidly declined. The maximum population density at 15°C, occurred at a light intensity of 80 ㎛ol m-2 s-1, whereas, the maximum population density was observed at a light intensity of 50 ㎛ol m-2 s-1 at 18°C. The growth rates of M. elongata gradually increased with increasing light intensity up to 50-80 ㎛ol m-2 s-1. The growth rate at the lower temperature (15°C) was higher than at the higher temperature (18°C) under light intensities less than 20 ㎛ol m-2 s-1. In contrast, the growth rate at a higher temperature (18°C) higher than at a lower temperature (15°C) under higher light intensities greater than 50 ㎛ol m-2 s-1. The maximum growth rates of M. elongata at 15°C and 18°C occurred at 80 ㎛ol m-2 s-1 and 50 ㎛ol m-2 s-1, respectively (Fig. 4).
Dense blooms of M. elongata were observed in early spring from a small eutrophic pond. M. elongata is one of the most common and widely distributed species of silica-scaled chrysophytes (Siver 1995, Kristiansen 2005). An investigation into the growth characteristics of individual species in the field as well as ecophysiological studies of selected species through laboratory culture experiments are needed to manage any algal bloom.
Water temperature, pH, conductivity and inorganic nutrients (PO4 and NO3) are major limiting factors affecting the distribution and growth of silica-scaled chrysophytes (Siver 1995, Kristiansen 2005). Si and light intensity are also important factors for development in this algal group (Sandgren et al. 1996, Saxby-Rouen et al. 1997, Kim et al. 2009).
On a global basis, M. elongata has been classified as a cold water organism (with an average weighted mean temperature ? 12°C), a high pH group (with weighted mean values above pH 7), and an organism that prefers eutrophic water (Siver 1995). However, a study on the growth of M. elongata regarding Si concentrations and light intensities has not been available until now. Additionally, culture studies concerning species-specific variations have rarely been performed, particularly with respect to the effects of nutrients (Si) and light intensity on growth of the genus Mallomonas.
Saxby (1990) observed that adding reactive Si to silicon deprived batch cultures increased growth rates in all experiments. In the present study, although the growth rate of M. elongata increased as the Si concentration increased up to 10 ㎛ol, growth rates were similar at all tested Si concentrations ranges. Overall, high population growth was exhibited at all ranges of Si concentrations between 0 and 40 ㎛ol. This result was similar to a report that the population growth and growth rates of three strains of M. caudata are not affected by Si concentration (Kim et al. 2009). The present study results support previous work that scale-bearing chrysophytes (S. petersenii) do not require large quantities of Si for growth, and that dramatic population growth can be achieved under severe Si-limited conditions (Sandgren and Barlow 1989, Kim et al. 2009).
In this study, M. elongata showed an inhibition of growth with light intensities greater than 80 ㎛ol m-2 s-1, and saturating light intensity at a lower temperature was higher than at a higher temperature. In contrast to that seen in Synura sphagnicola (Healey 1983), the growth rates of M. elongata increased at the same light intensity as that which increased culture temperature. Moreover, the growth characteristics of M. elongata under two experimental temperature conditions showed similar results to our previous study (Lee et al. 2007). These results were similar to reports of ours and other studies showing that growth inhibition appeared in certain strains of S. petersenii and M. caudata under high light intensity (Meeson and Sweeney 1982, Kim et al. 2008, 2009). The characteristics of M. elongata shown in this study support our previous reports that planktonic silica-scaled chrysophytes have strain-specific growth characteristic based on individual environment factors such as temperature and pH, nutrients and light intensity (Kim et al. 2008, 2009).
Previous studies (Lee et al. 2005, 2007) have shown that two strains of M. elongata isolated from different water bodies have significantly different growth characteristics under the same temperature condition (18°C). The optimum growth of the strain that we used in this study appeared at 15°C, whereas very low population growth was observed at 18°C. The other strain isolated from a different reservoir showed high population growth and growth rates at both 15°C and 18°C. In the present study, the same strain isolated from Jido pond located in Kyungpook National University, which showed very low growth rates in a previous study, displayed a higher growth rate at 18°C than that at 15°C at light intensities greater than at 50 ㎛ol m-2 s-1. This could have resulted from differences in pH.
In conclusion, these results supports previous reports in that each strain among the same silica-scaled chrysophytes species isolated from different water bodies has strain-specific growth adaptations for various environmental factors. These results suggest that population development and succession of silica-scaled chrysophytes including M. elongata could be governed by individual factors as well as an interaction between various factors such as temperature, pH, nutrients, and light intensity. Further culture studies on the influence of the interaction between these environmental factors on growth and development of individual species of this algal group will be required to fully understand how to controll and manage algal blooms.