A number of dinoflagellates cause harmful algal blooms (HABs) in the marine ecosystem. Some have complex life cycles, including the benthic cyst stage. Dinoflagellate resting cysts play important ecological roles in bloom initiation and termination, as agents of survival and dispersal, in genetic recombination, and as a potential toxin source to benthic mollusks. Thus, geographical cyst mapping pinpoints the presence of “seed populations”for bloom initiation sites and traces dispersal paths that suggest the potential for future HAB outbreaks (Pfiester and Anderson 1987).
The Yellow Sea forms one of the largest epicontinental shelves. It is partially enclosed by the Chinese and Korean coasts and borders on the Bohai and East China Seas. The Yellow Sea rests in a tectonically stable trough that was submerged during the postglacial sea-level rise, with an average water depth of 55 m (Jin and Chough 1998, Uehara and Saito 2003).
Yellow Sea sediments consist of sand, muddy sand,sandy mud, and mud, in order of the percentage of mud or sand. The suspended fine-grained materials near shore move along the Yellow Sea circular current system route and are transported to and accumulate on the seafloor at the eddy center of the Yellow Sea to form muddy sediments. As a result, the largest mud-rich sediment patch developed in the central part of the Yellow Sea, and muddy sand and sand are distributed in an outward manner (Park and Khim 1992, Uehara and Saito 2003, Shi et al. 2004).
Several dinoflagellate cyst distribution studies have been conducted in the East China and / or Yellow Seas (Qi et al. 1996, Cho and Matsuoka 2001, Cho et al. 2001). However, the previous studies only covered part of the Yellow Sea; thus, they are insufficient for interpreting cyst distributions over the entire Yellow Sea. In this study, we investigated the spatial distribution and species composition of dinoflagellate cysts in surface sediment samples, which covered nearly the entire Yellow Sea continental shelf. The cyst distribution was compared to hydrographic and sedimentary properties of the Yellow Sea to understand the origin, dispersal paths, and depositional sites of the cysts.
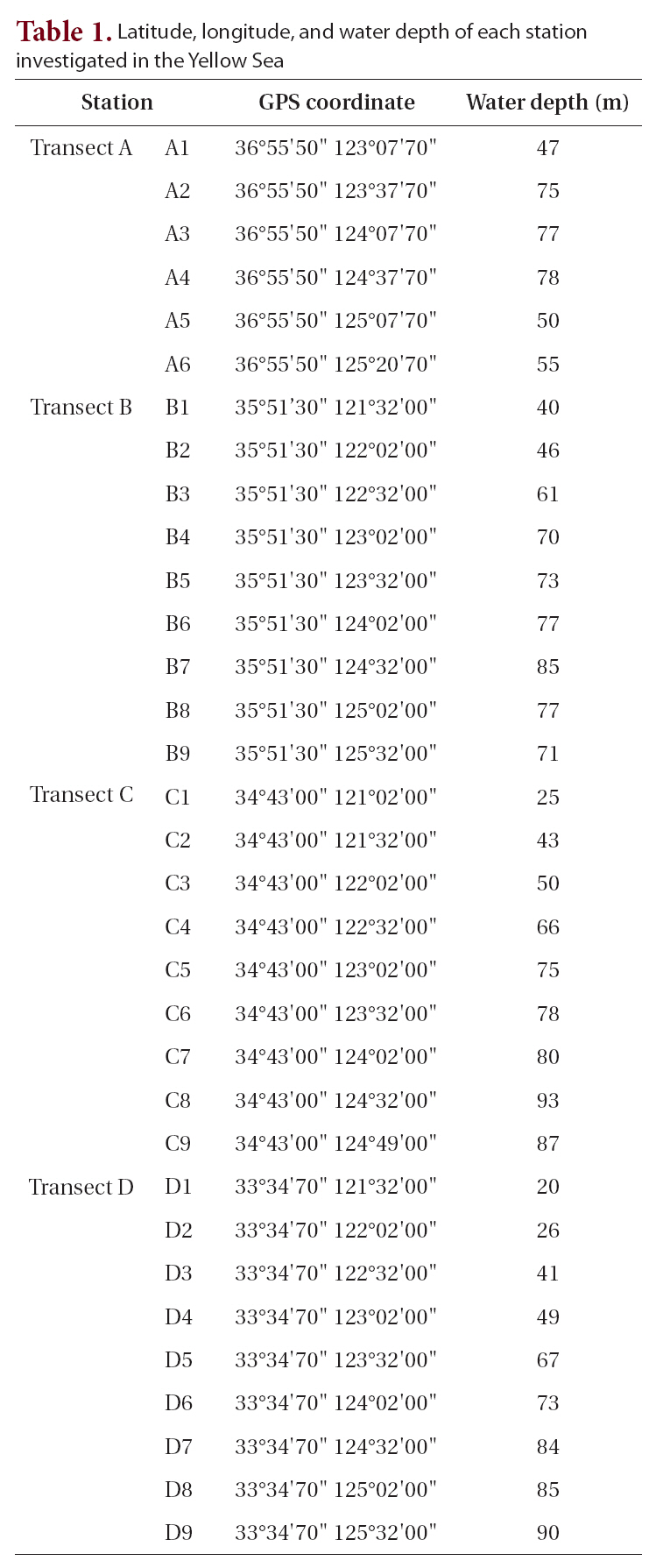
A cruise was conducted around the Yellow Sea for this study (Table 1) on October 15-31, 2003 using the
After calculating water content, sediment samples were processed according to Matsuoka and Fukuyo (2000). Briefly, they were treated with 10% HCl and 47% HF to dissolve calcium carbonate and silicate materials, respectively. They were then rinsed with distilled water and sonicated using an Ultrasonic Cleaner 5210 (Branson Ultrasonics, Danbury, CT, USA) for 30 s. After straining through 120 ㎛ and 20 ㎛ mesh sieves, the refined sediments remaining on the 20-㎛ sieve were transferred to a 15 mL tube and suspended in 10 mL of distilled water. A 1 mL aliquot of each sample was observed 42under an inverted light microscope (Axiovert 200; Zeiss, Oberkochen, Germany). Cyst concentrations were calculated as the number of cysts per gram of dry weight.
Identification followed Matsuoka and Fukuyo (2000).
[Table 1.] Latitude longitude and water depth of each station investigated in the Yellow Sea
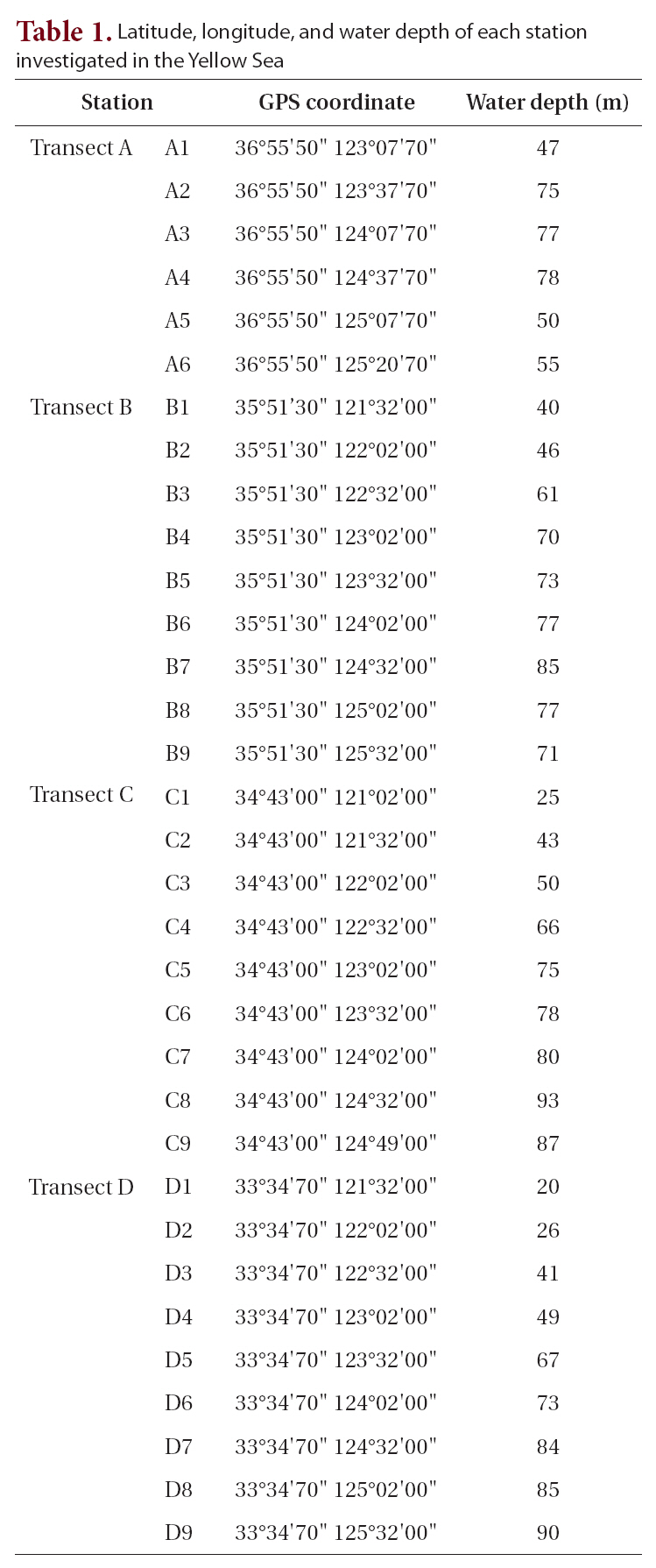
Latitude longitude and water depth of each station investigated in the Yellow Sea
Resting cysts were called cysts for the sake of brevity, and only living cysts were counted.
Twenty-five different types of dinoflagellate cysts were identified in Yellow Sea surface sediments, representing 14 genera and belonging to the Gonyaulacales (10 species), Peridiniales (10 species), and Gymnodiniales (4 species) (Table. 2-5). The most common species were
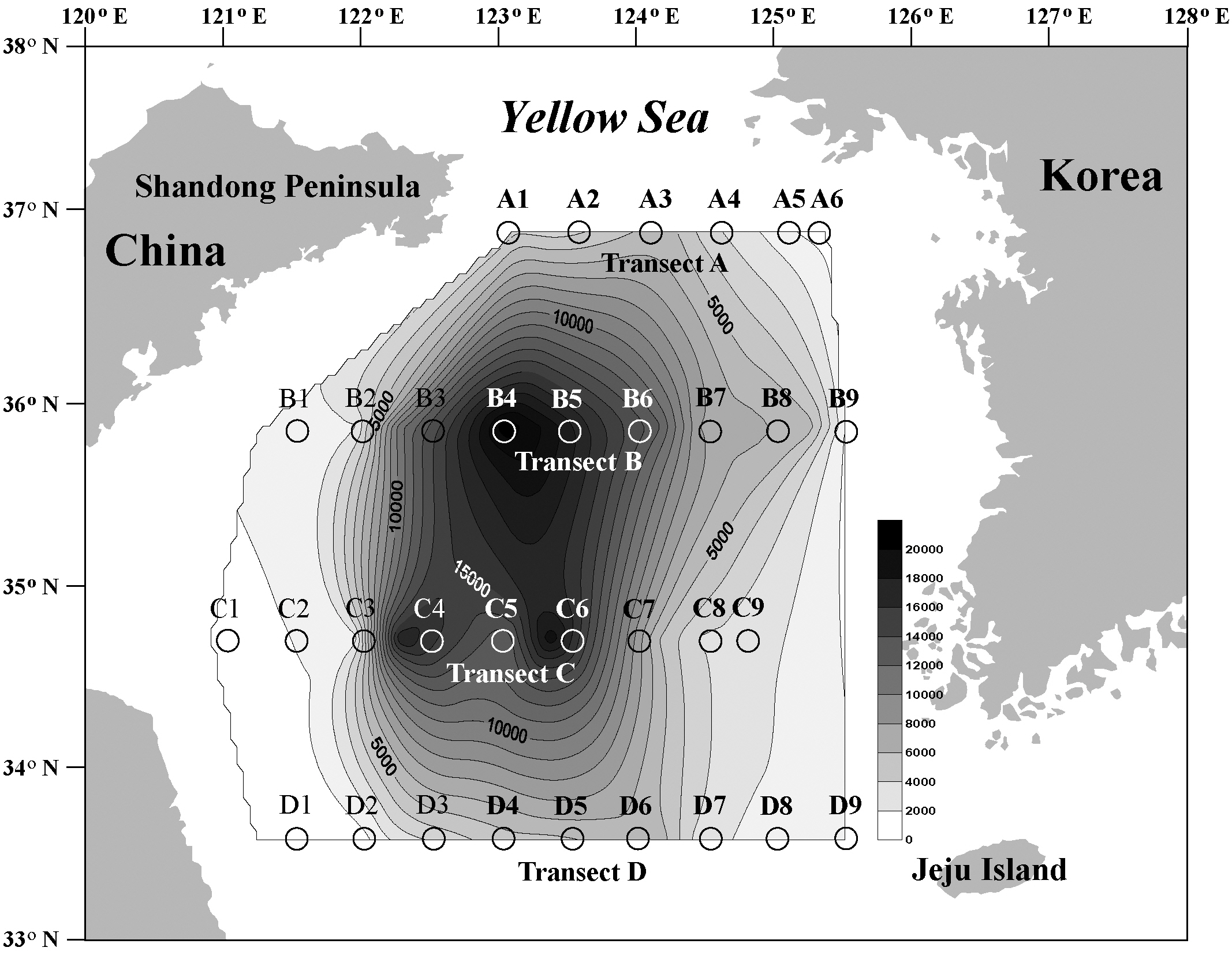
Transect A was the northernmost transect located off the Shandong Peninsula, and 23 types of cysts were iden-
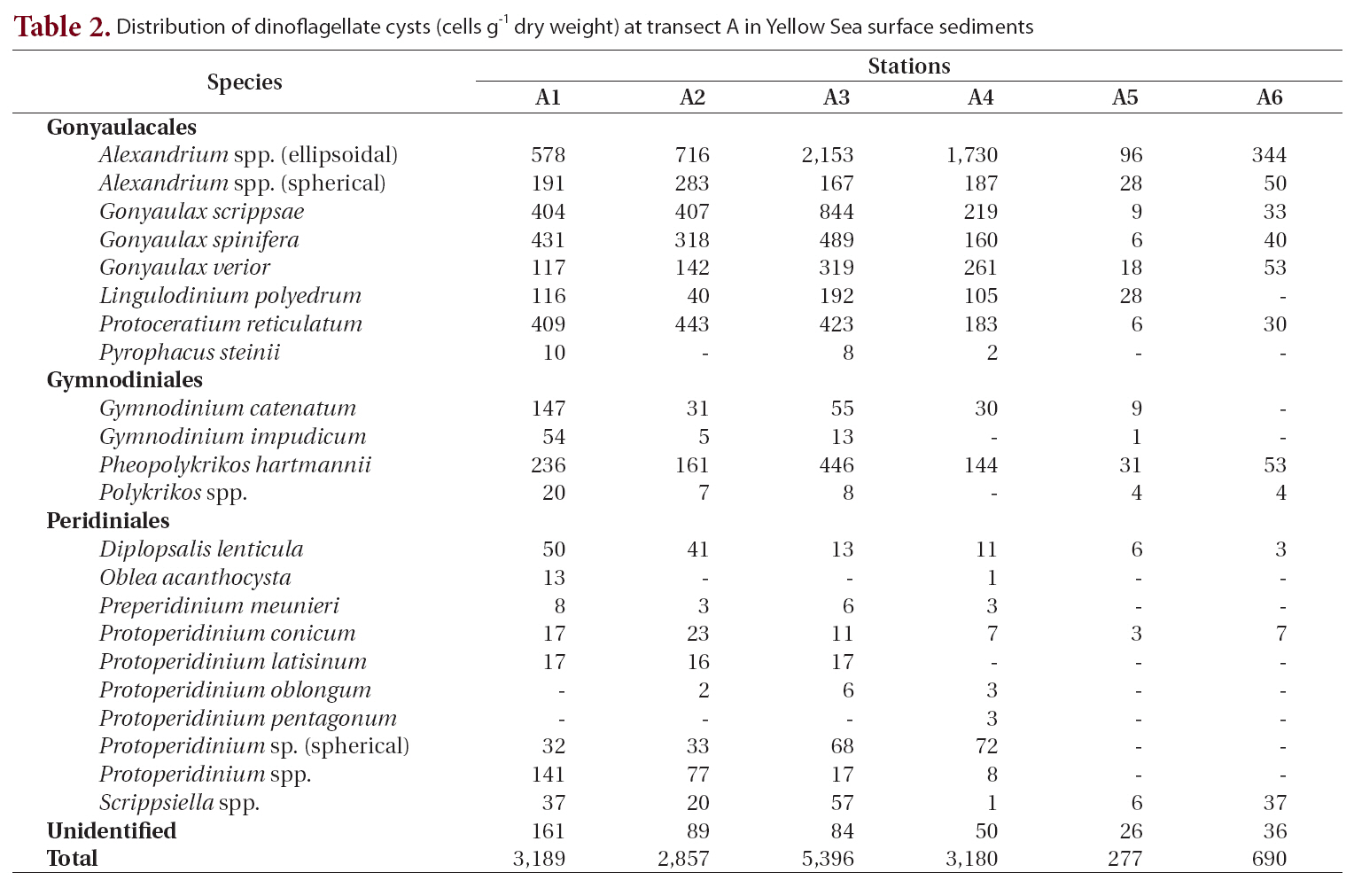
tified (Table 2, Fig. 1). Cyst concentrations were significantly higher at station A3 (5,396 cells g-1 dry weight) in the central area than at the stations in the marginal areas of the Shandong Peninsula and Korea. Alexandrium spp. (ellipsoidal type, 96-2,153 cells g-1) were the most abundant, followed by
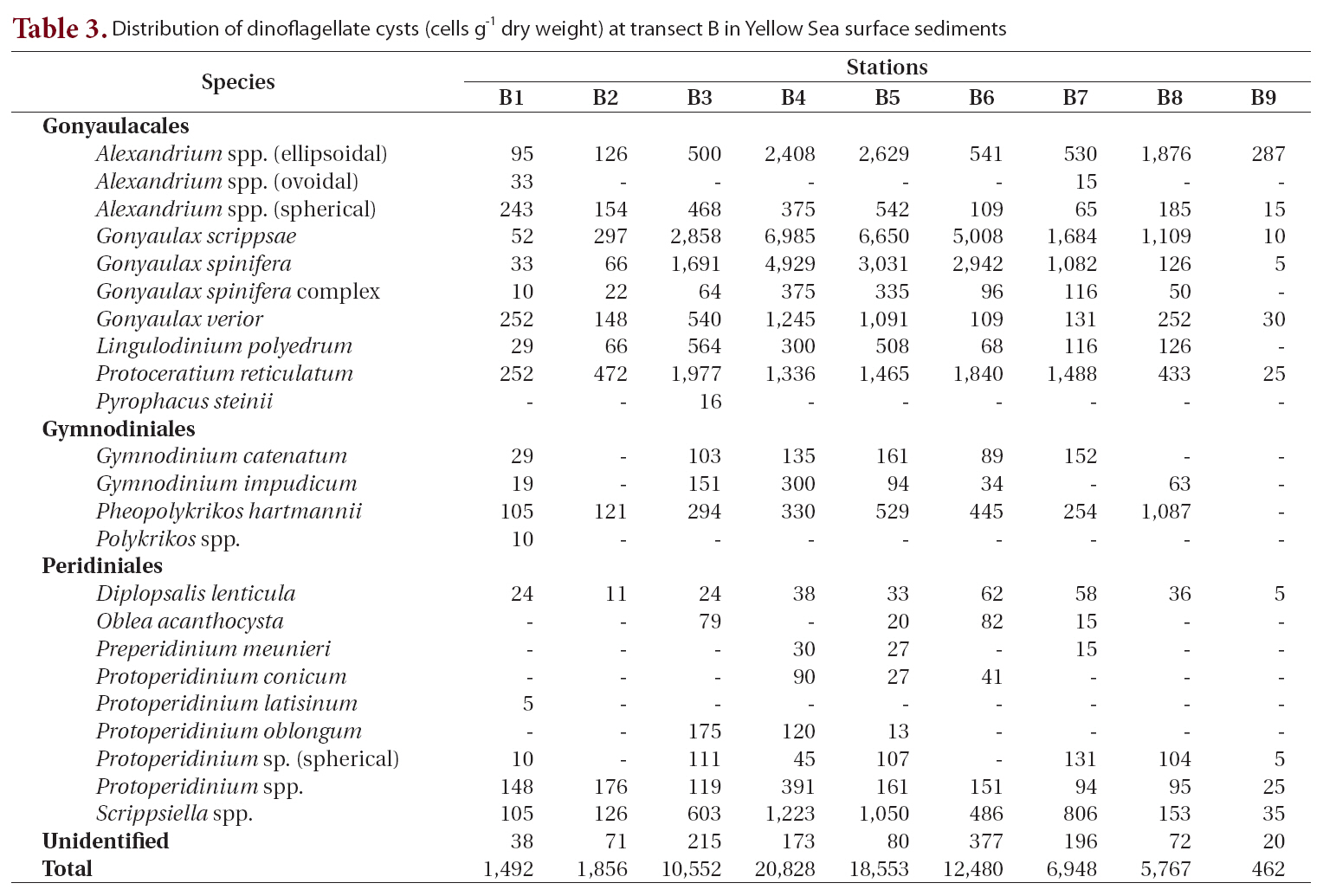
We recorded 24 types of cysts from transect B located south of transect A (Table 3, Fig. 1). Total concentrations were markedly high in the central areas (stations B3-B6, 10,552-20,828 cells g-1) but decreased sharply towards the coastal areas of China and Korea. Station B4 in the central region had the highest density of cysts (20,828 cells g-1) among all sediment samples analyzed. The major species identified were
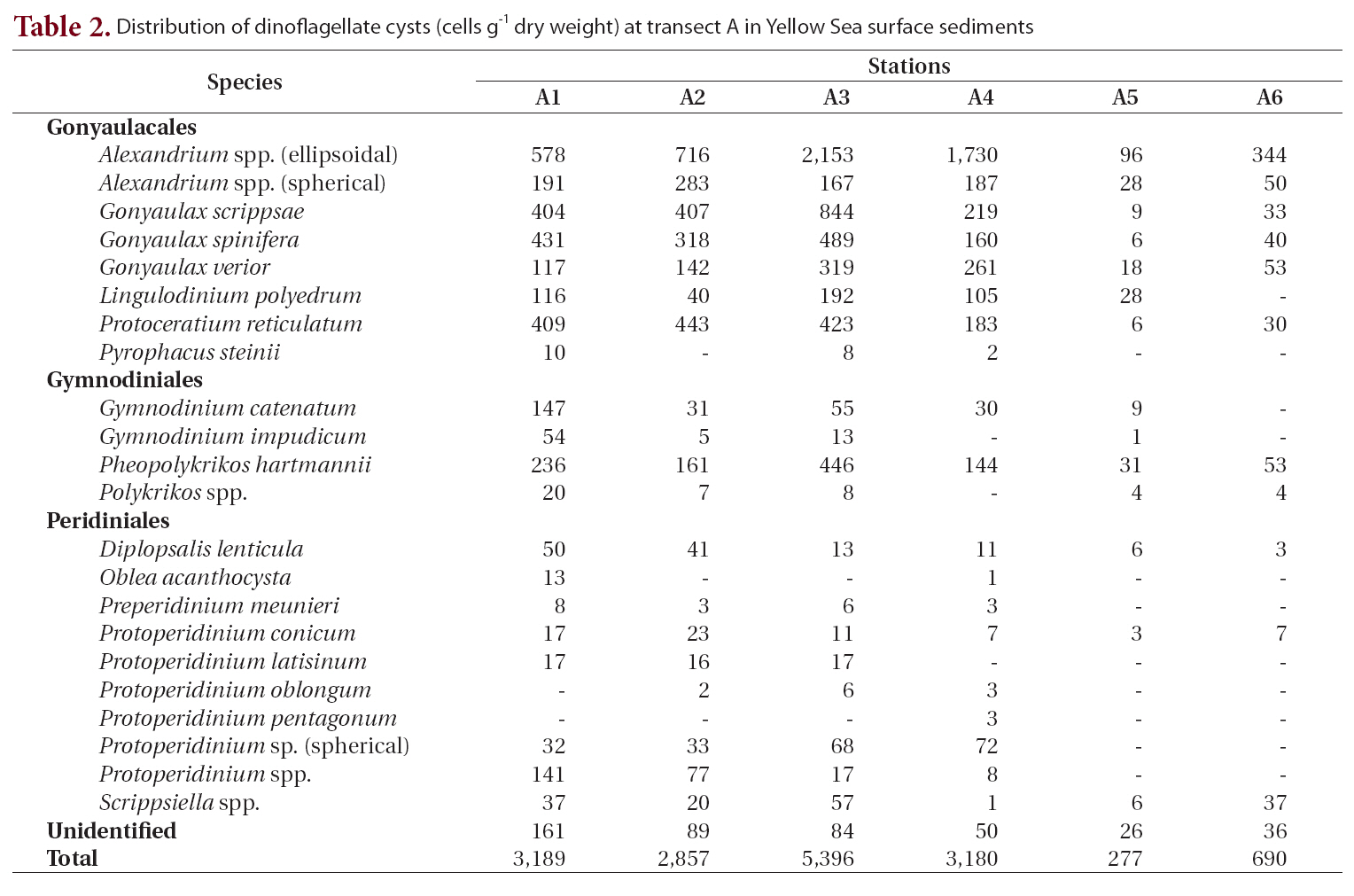
Distribution of dinoflagellate cysts (cells g-1 dry weight) at transect A in Yellow Sea surface sediments
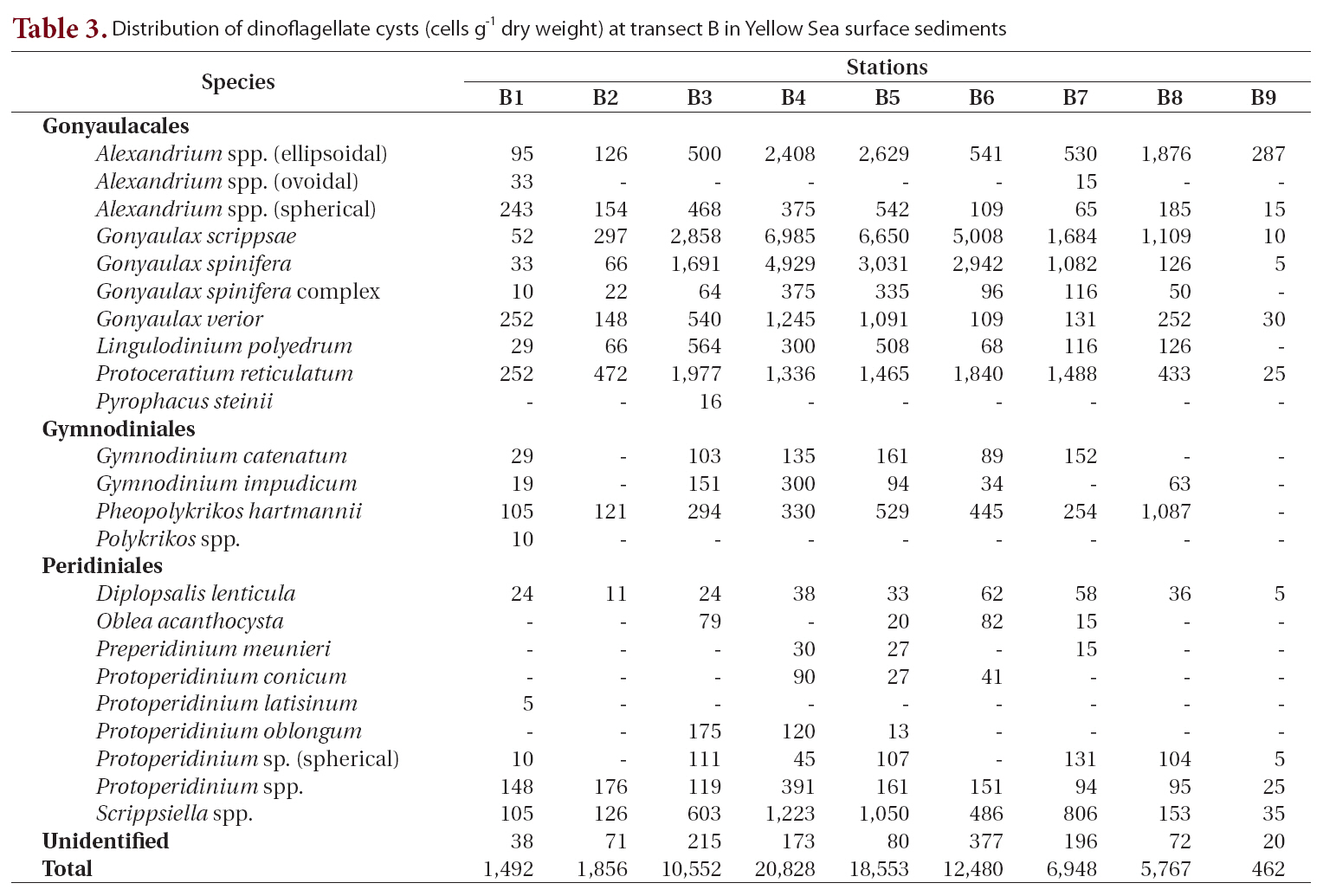
Distribution of dinoflagellate cysts (cells g-1 dry weight) at transect B in Yellow Sea surface sediments
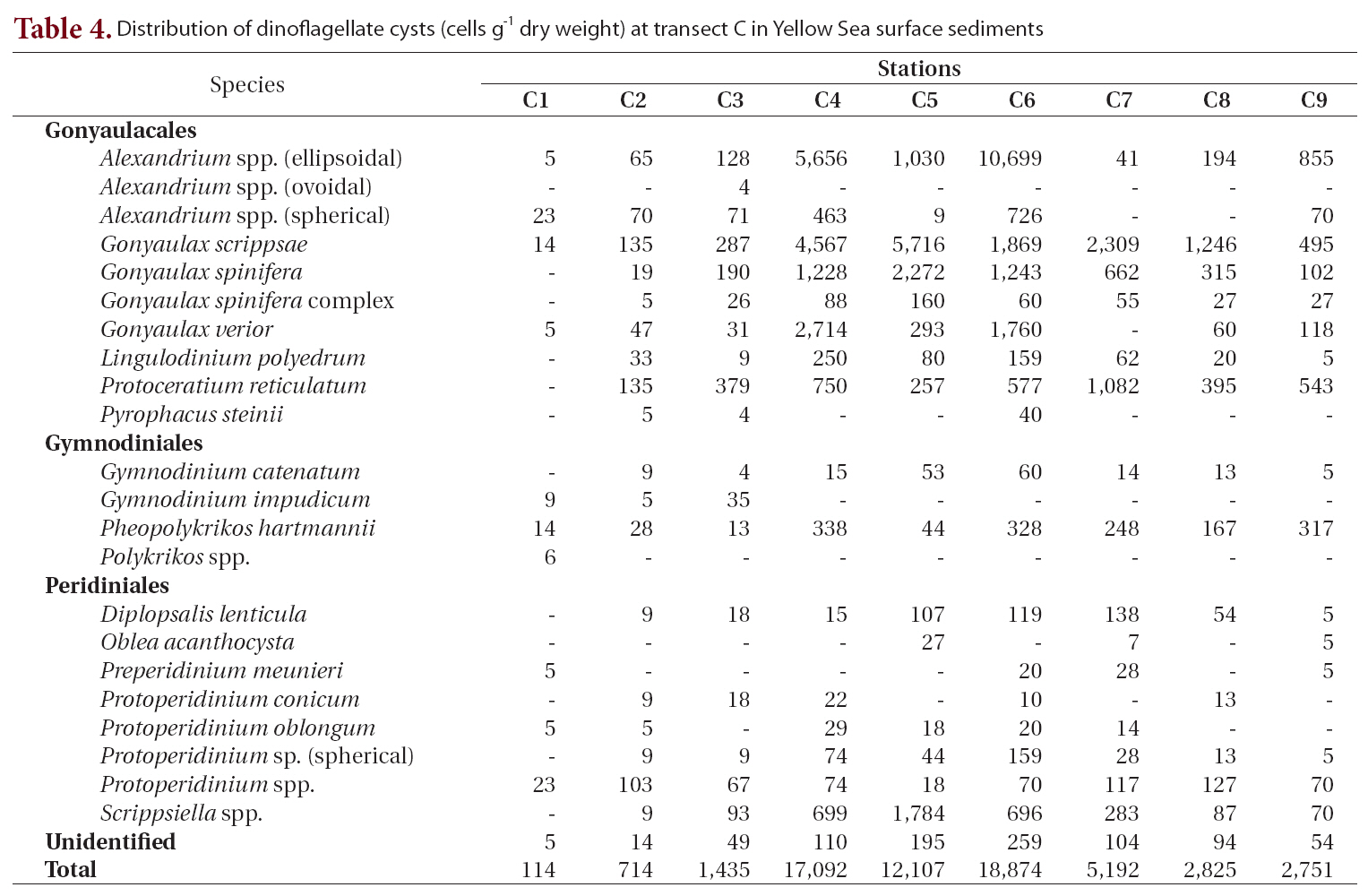
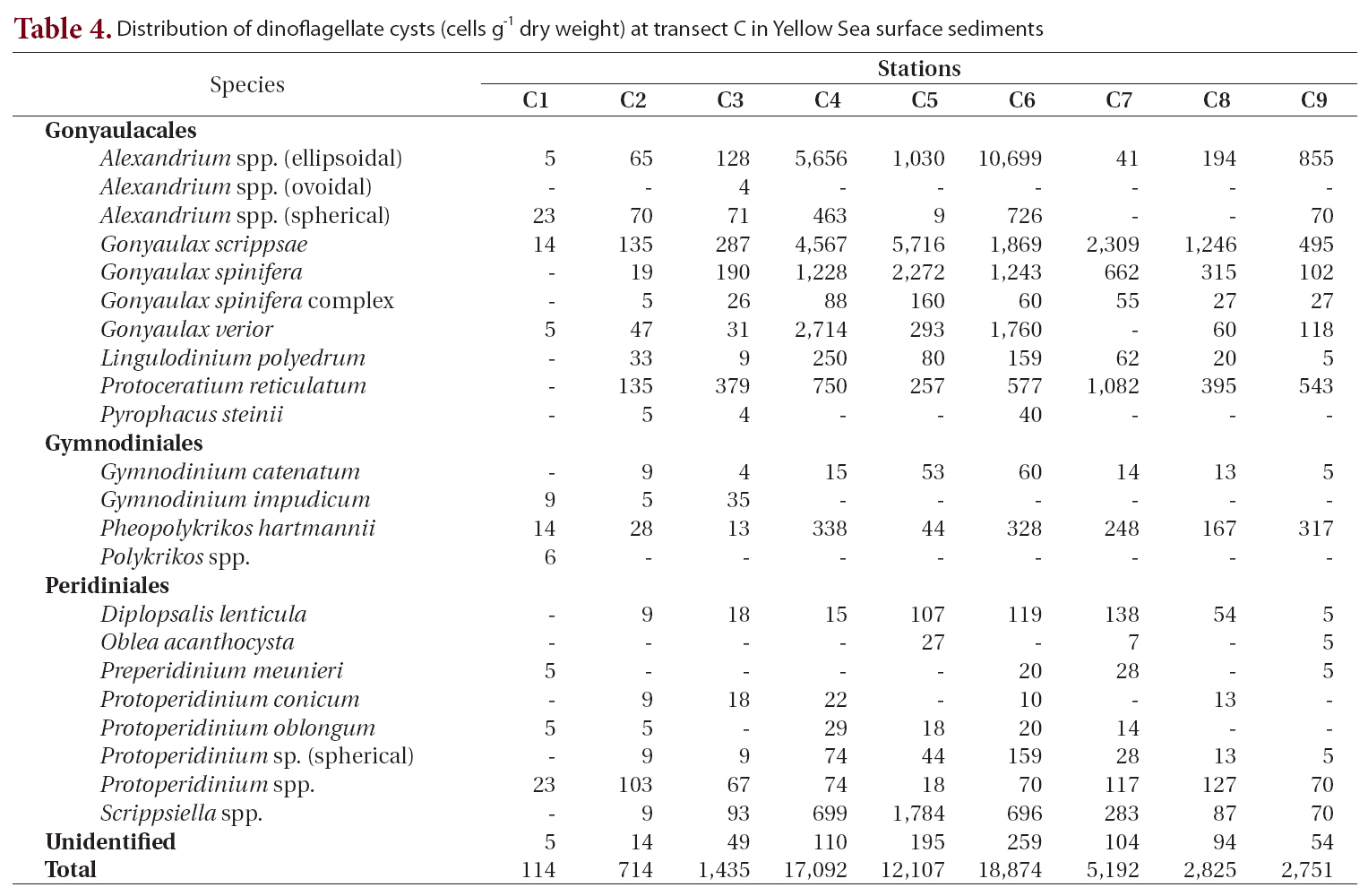
Distribution of dinoflagellate cysts (cells g-1 dry weight) at transect C in Yellow Sea surface sediments
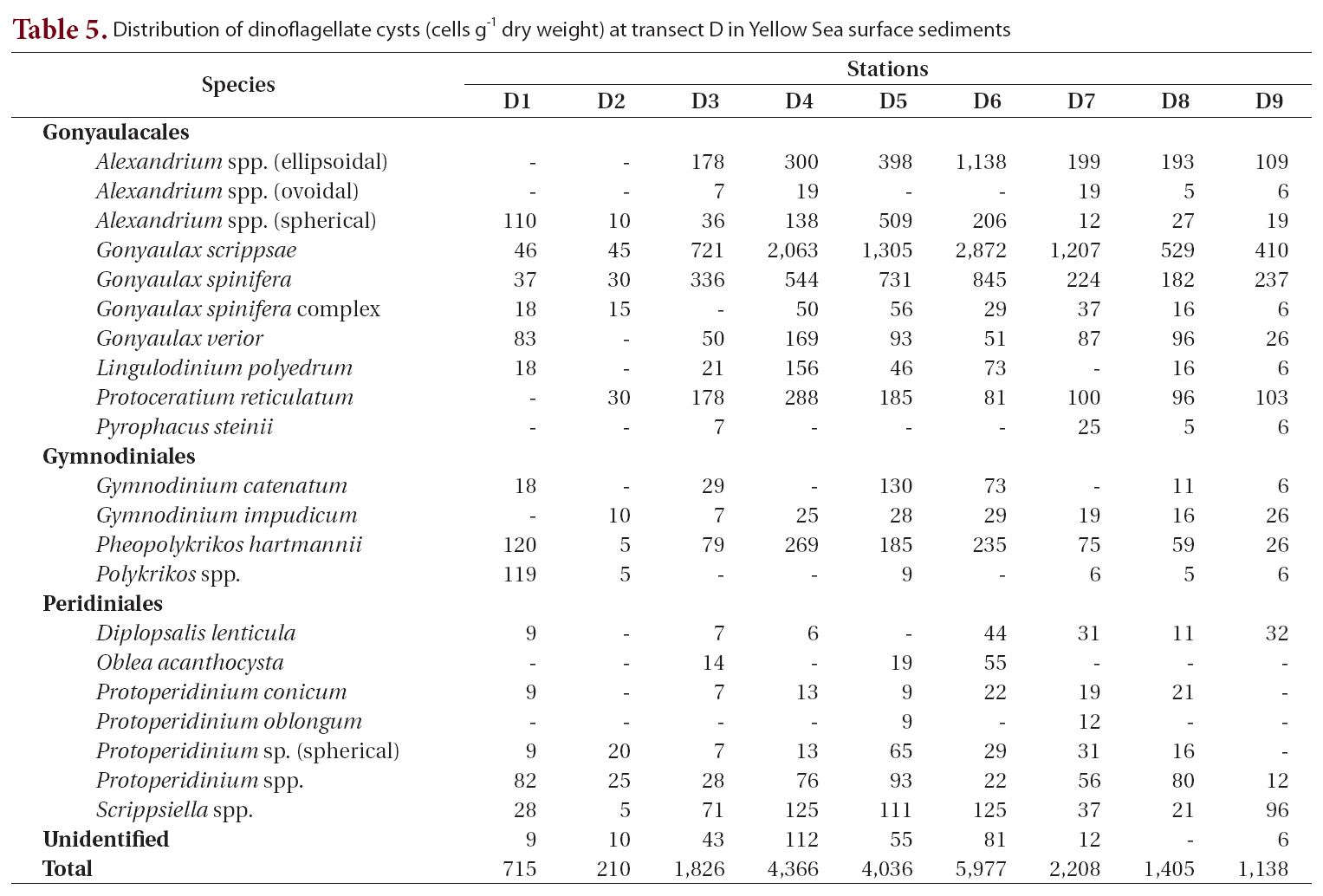
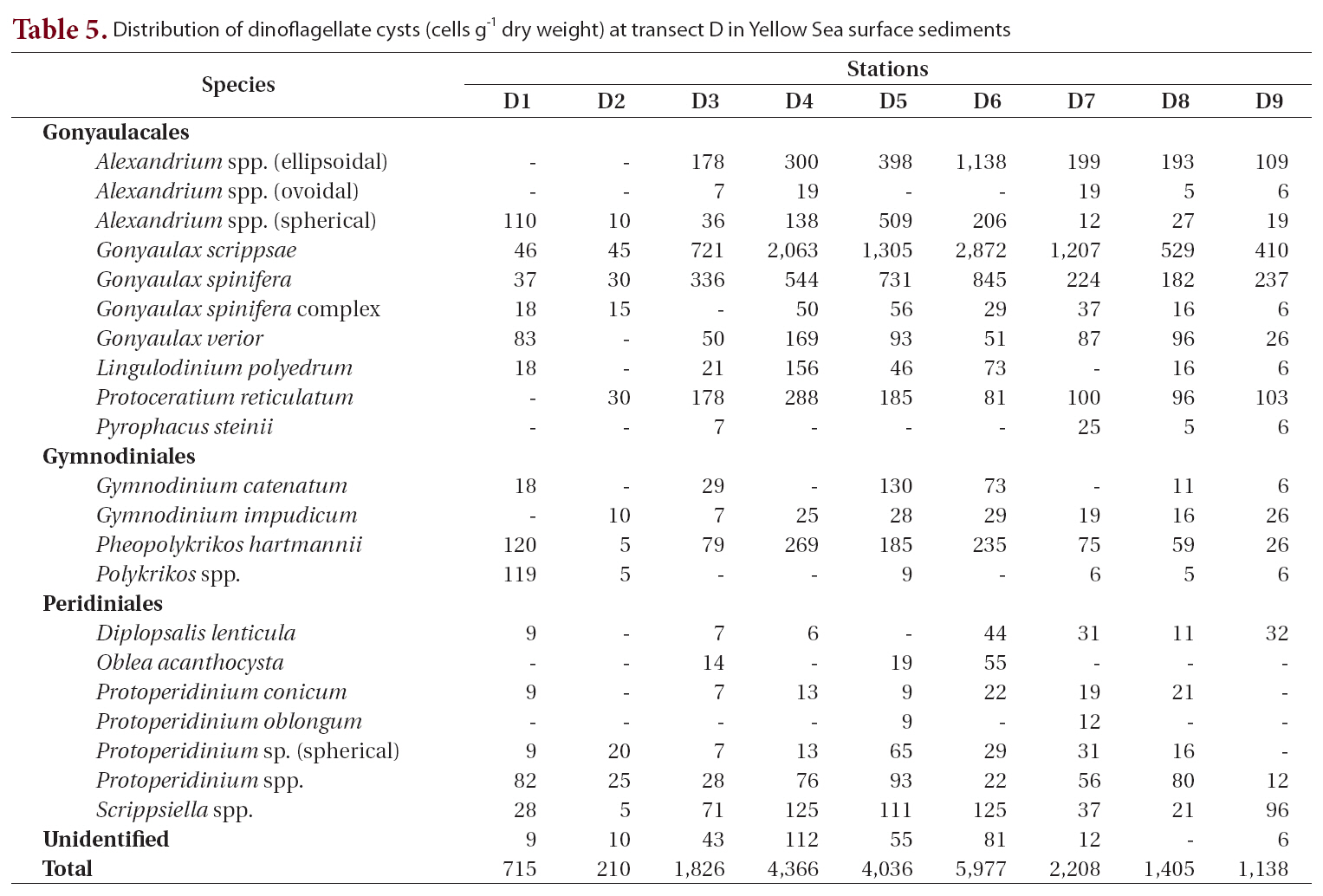
Distribution of dinoflagellate cysts (cells g-1 dry weight) at transect D in Yellow Sea surface sediments
dal type, 95-2,629 cells g-1), and
Twenty-three types of cysts were encountered at transect C, which was between transect B and the southernmost transect D (Table 4, Fig. 1). Cyst concentrations were high in the central areas (stations C4-C6, 12,107-18,874 cells g-1) and decreased sharply toward the marginal areas of both the Chinese and Korean coasts. The lowest density among all sediment samples analyzed was recorded at station C1 on the Chinese coast (114 cells g-1). The highest value was 18,874 cells g-1 at station C6, where
Transect D was the southernmost transect located off Jeju Island, and 22 cyst types were identified (Table 5, Fig. 1). The offshore stations (stations D4-D6, 4,036-5,977 cells g-1) showed much higher cyst concentrations than did the nearshore stations (Fig. 1). The most dominant species was
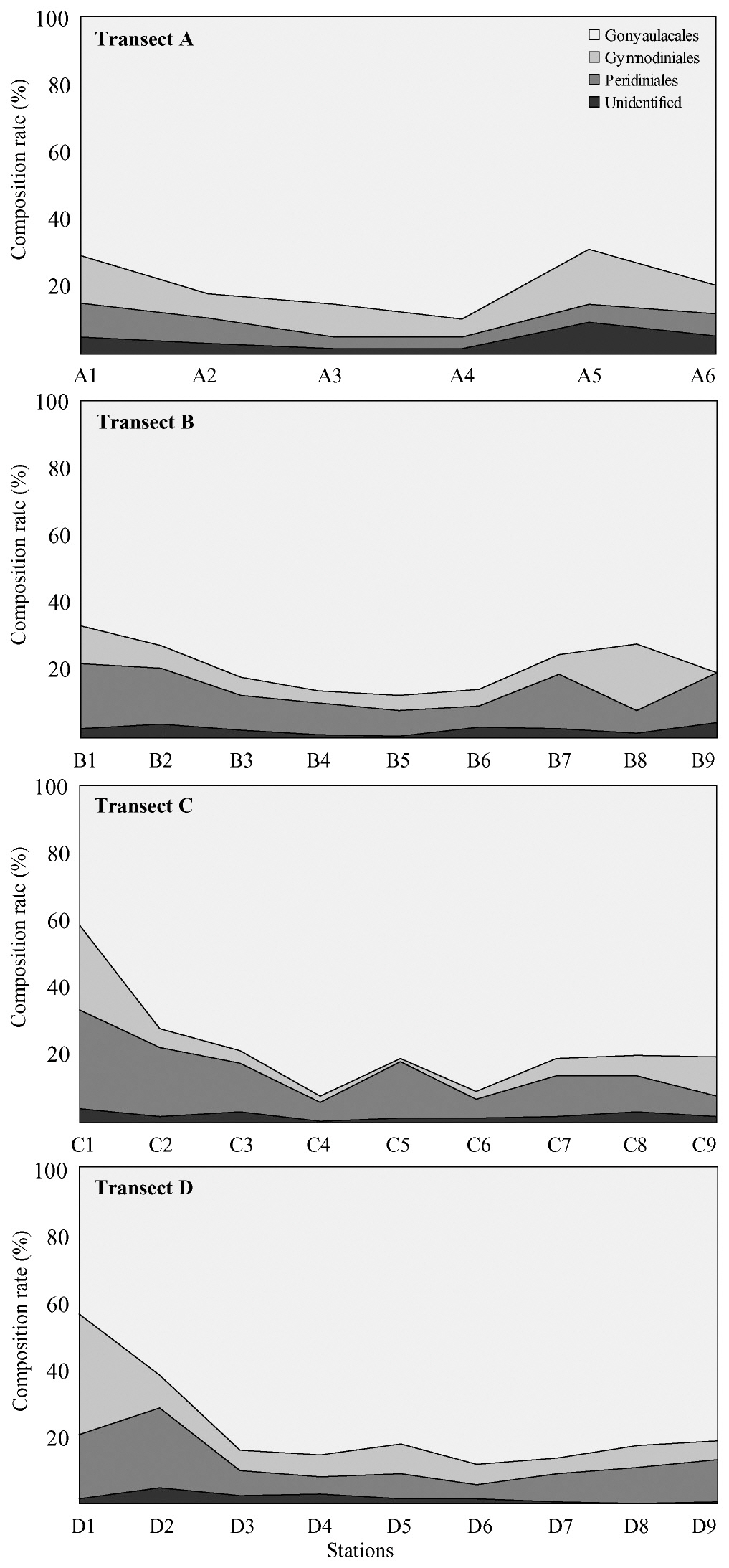
Cyst densities were generally highest near the offshore center of the Yellow Sea and gradually decreased towards both the Chinese and Korean coasts within each transect (Fig. 1). The middle transects B and C showed higher concentrations than did the northern transect A and the southern transect D. However, the trend of a gradual decrease towards the higher and lower latitudes was less prominent than that towards both nearshore directions. Cyst concentrations on the Chinese coast
Dinoflagellate cysts belonging to the Gonyaulacales generally comprised over 50% of all cysts collected, but two exceptions of an abrupt proportional decrease in Gonyaulacales at stations C1 and D1 on the Chinese coast were noteworthy (Fig. 2). The proportional density of the Protoperidiniales tended to gradually increase toward the Chinese and Korean coasts, whereas total cyst concentrations decreased dramatically.
The 33 sediment samples surveyed in this study provided dinoflagellate cyst records from nearly the entire Yellow Sea continental shelf. Cho and Matsuoka (2001) investigated the cyst spatial distributions in the East China and Yellow Seas, but their study areas covered only parts of the Yellow Sea; thus, they provided limited information on dinoflagellate cyst biogeography. The sampling stations surveyed in our study covered wide areas along four latitudinal transacts that connected the Chinese and Korean coasts, of which the northernmost reproductransect was level with the Shandong Peninsula and the southernmost was level with Jeju Island.
Cyst concentrations were generally markedly elevated around the center of the Yellow Sea and gradually decreased in all four outward directions. This result was congruent with Cho and Matsuoka (2001) who investigated the spatial distributions of cysts in the East China and Yellow Seas and noted unusually high cyst concentrations in the offshore center of the Yellow Sea. Our result of high offshore cyst concentrations in the Yellow Sea were also comparable to those of Cho et al. (2003) who reported that the number of cysts at offshore stations was approximately four times higher than that at inshore stations in the southern Korea Sea. This tendency toward increased cyst abundance in an offshore direction has also been well documented in previous studies (e.g., White and Lewis 1982, Dale et al. 2002). The total number of cysts recorded around the offshore center of the Yellow Sea was much higher than that of previous studies from the Korean coast (Cho and Matsuoka 2001, Park and Yoon 2003, Park et al. 2004).
The distribution of dinoflagellate cysts is governed
not only by environmental factors such as sediment particle size, sedimentation rate, and hydrographic and geographical features but also by biological factors (Dale 1976, White and Lewis 1982, Turgeon et al. 1990). Cysts behave like fine-grained sedimentary particles and move and concentrate along hydrodynamic systems.
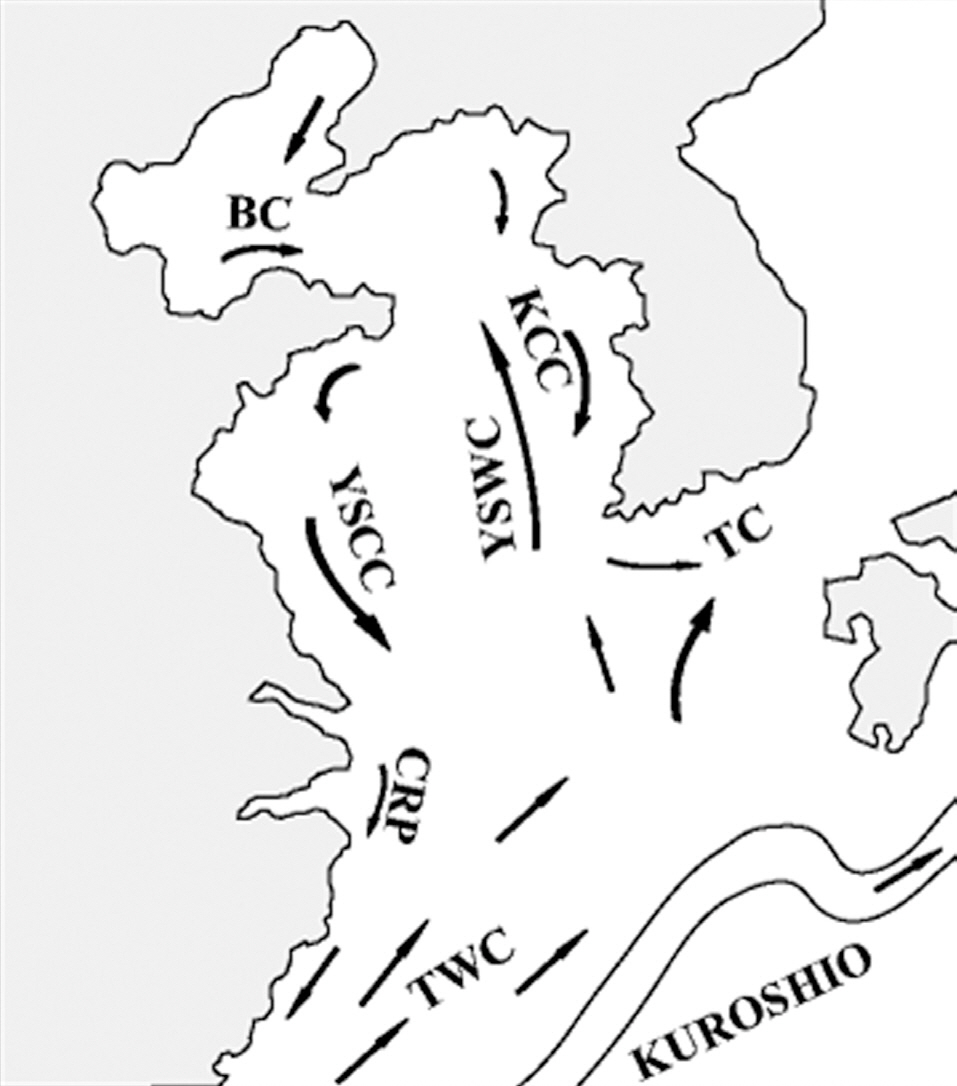
The general hydrographic features of the Yellow Sea are characterized by the northward inflow of the Yellow Sea Warm Current and the subsequent southward flow along the Chinese and Korean coasts (i.e., the Yellow Sea Coastal Current and the Korean Coastal Current, Fig. 3). These coastal currents are compensated for by the northward flow of the Yellow Sea cold bottom water along the Yellow Sea trough, which forms a large cyclonic (counterclockwise) eddy in the central part of the Yellow Sea (Su 1998, Shi et al. 2004). Thus, surface sediment distributions are under the direct influence of the centrifugal force of this current movement, and the largest fine-grained muddy deposits are located in the offshore central Yellow Sea (Park and Khim 1992, Uehara and Saito 2003). Shi et al. (2004) demonstrated that the net transport direction of sediments in the Yellow Sea is toward the centrally located fine-grained sediment deposits. This sediment transport pattern is also consistent with several tracers of sediment sources such as the distribution of total suspended matter concentrations, the δ13C value of particulate organic carbon, values of polycyclic aromatic hydrocarbons (Shi et al. 2004), and various geochemical elements (Kim et al. 1998). These sedimentary materials originated and were dispersed from the old- and present-day Huang He (Yellow River) system (Alexander et al. 1991, Park and Khim 1992, Shi et al. 2004).
Our results showing concentric high-density cyst concentrations in the Yellow Sea were also congruent with these sedimentary properties, which were strongly correlated with water depth and the current circulatory system. Thus, high cyst concentrations in the offshore central Yellow Sea can be attributed to the prevailing Yellow Sea circulation associated with the transportation and deposition of fine-grained sediments. A tendency for an increase in the relative abundance of cysts along a deep-sea gradient may be largely influenced by larger scale offshore transport of neritic and estuarine cysts to deeper depositional sites (Dale et al. 2002).
Other than the underlying possibility of an exogenous origin for most benthic cysts in the central Yellow Sea, there are also possibilities for endogenous origins, e.g., offshore migration of neritic dinoflagellate bloom populations or vertical sinking of
Ellipsoidal
Marine environmental conditions can be estimated by changes in total cyst productivity and proportional changes in a particular taxon (Dale et al. 1999, 2002, Matsuoka 1999). For example, a high relative abundance of heterotrophic protoperidinioid cysts occurs in nutrient-enriched areas such as in upwellings or eutrophicated coastal areas (Dale et al. 2002). The proportion of heterotrophic cysts belonging to the Protoperidiniales was lowest in the central Yellow Sea and gradually increased toward the Chinese and Korean coasts along with a dramatic decrease in total cyst concentrations. The significant proportional increase in protoperidinioid cysts towards the Chinese coast is noteworthy and may imply that environmental conditions at these coastal areas differ from conditions at other areas. However, the proportions of heterotrophic protoperidinioid cysts on the Chinese and Korean coasts of the Yellow Sea are lower than other coastal areas (e.g., Matsuoka et al. 2003, Shin et al. 2010). However, this interpretation may be arbitrary due to transport and dispersal of sedimentary materials containing microflora by the prevailing hydrographic system mentioned above.
Among 2,000 extant dinoflagellate species, approximately 5% are known to have a corresponding cyst stage in their life cycle (Matsuoka and Fukuyo 2003). Cyst morphologies of many notorious HAB species such as