Many plant species are dispersed by herbivorous animals(e.g., Welch 1985, Malo and Suarez 1995, Pakeman et al. 2002). Several studies have indicated that large herbivores act as potentially important seed dispersal agents(Gill and Beardall 2001, Myers et al. 2004, Cosyns and Hoffmann 2005, von Oheimb et al. 2005). After ingestion by large herbivores, seeds undergo chemical and physical attacks such as chewing, rumination, and digestion.Small, hard-coated seeds with no obvious specialized diasporas are less likely to be damaged during passage through the digestive tract of ruminants and are more likely to be dispersed in dung, whereas larger seeds are heavily damaged (Janzen 1984, Gill and Beardall 2001,Bruun and Fritzbøger 2002, Heinken et al. 2002, Pakeman et al. 2002, Myers et al. 2004, Mouissie et al. 2005).The number of germable seeds in dung varies seasonally,and this seems to reflect seed production at the grazing site (Malo and Suarez 1995). However, previous studies on seed dispersal have focused primarily on the results of germination experiments with seeds excreted by herbivores (e.g., Malo and Suarez 1995, Pakeman et al. 2002, Cosyns and Hoffmann 2005). Such approaches may underestimate both species and seed numbers (Couvreur et al. 2004). The dung of herbivores may also contain many seeds that cannot germinate as a result of differences in germination conditions among plant species (Grime et al. 1988, Ishikawa 2011) and/or in seed conditions such as immaturity, death, or dormancy. Grazing of immature seeds (including flower buds and flowers) or death of seeds by passing through the digestive tract may preclude the next generation. Thus, seed dispersal by herbivores can involve negative effects on plant populations and communities.
The sika deer
Plant species growing in short grasslands produce reproductive organs on short stems close to the leaves, and the reproductive organs are very small and intermingled with foliage. The sika deer is a grazer (Hofmann 1985), and the reproductive organs are thus likely to be ingested by the deer together with the leaves and stems at any maturity stage in the short grasslands. If sika deer continually and nonselectively graze various herbaceous plants at different developmental stages in the community, two hypotheses arise on the fate of seeds grazed by the deer: (i) sika deer dung may contain many small seeds of various herbaceous species growing at the grazing site, and (ii) immature seeds grazed by sika deer together with germable seeds may disappear during passage through the digestive tract.
The objectives of this study were to test these hypotheses by (i) comparing species composition and abundance of each species at the grazing site with those in sika deer dung and (ii) comparing the seasonal traits of production and maturity of reproductive organs (inflorescences) of dominant plant species in the community at the grazing site with those of the abundance and maturity of seeds in sika deer dung on a temperate grassland established in an urban park, Nara Park. At the same study site, Ishikawa (2010) suggested that although mature seeds of the dominant
Nara Park (approximately 660 ha) is a large urban park, which is located in the city of Nara (34°40′ N, 135°50′ E) in western Japan. The area has a warm-temperate climate with a mean annual rainfall of 1,338 mm. Mean annual temperature is 14.6°C, with an average monthly minimum of 3.8°C in January and a maximum of 26.6°C in August (1971-2000) (Japan Meteorological Agency 2011). The climax vegetation in this area is a lucidophyllous forest of
In Nara Park, sika deer have a home range of approximately 12 ha for females and approximately 8 ha for males and show daily activity throughout this range (Miura 1977). The deer move between open land and forest and graze in the grasslands and rest in the forests (Fukunaga and Kawamichi 1975, Fukunaga 1976). The deer have long been protected for religious reasons, and they have no natural predators. During the 1940s, the deer population in the park declined to no more than 80 individuals as a result of poaching to provide food during the war, but by 1965 it had recovered to approximately 900, and has ranged from 1,000 to 1,300 for the last 30 years (Foundation for the Protection of Deer in Nara Park 2011). The current population level appears to be at or close to the park’s carrying capacity (Torii and Tatsuzawa 2009). As a result of heavy deer impacts for many years, Nara Park has a unique ecosystem and a distinctive landscape; large open areas are covered with
This study was conducted in the Tobihino area (approximately 1.9 ha), which is a flat part of southeastern Nara Park (Ishikawa 2010, 2011). The deer density is particularly high (between 430 and 5,500 individuals/km2) (Foundation for the Protection of Deer in Nara Park unpublished data) in the Tobihino area. The Tobihino area is covered with grasslands dominated by
>
Census and analysis of vegetation
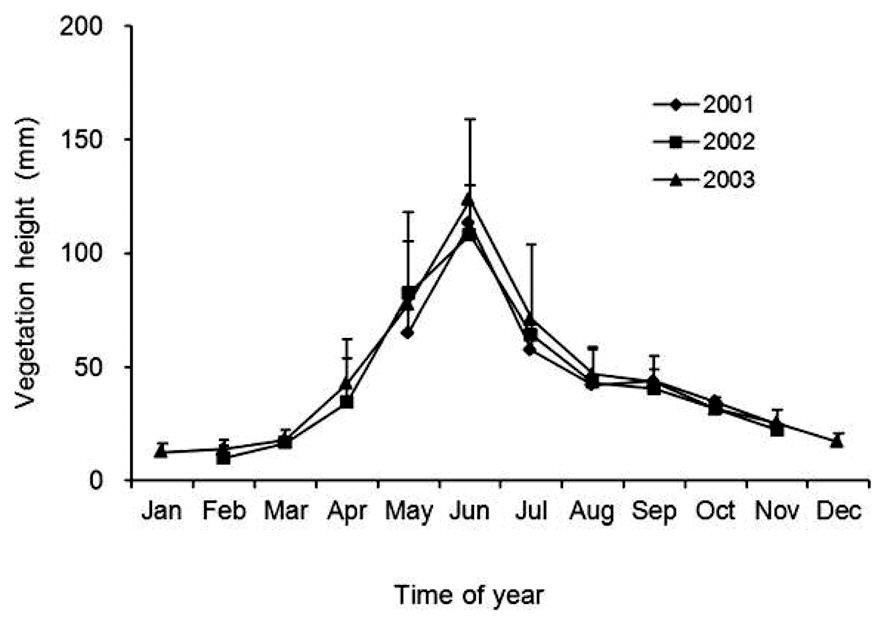
Censuses for plant species composition and percent cover of each species present were performed from May 2000 to December 2003. Vegetation height was assessed from May to November 2001, February to November 2002, and January to December 2003. Five permanent circular plots (each 10 m2) were established along a straight line for periodic vegetation censuses. There was a separation of 10 m between the center of one plot and the center of the next plot. In each plot, two quadrats (each 50 cm × 50 cm) were established randomly during each census. Thus, at each census, 10 quadrats were assessed for vegetation height, plant species composition, and the percentage cover of each species present in the plot.
Vegetation height, as an index of the vegetation successional stage, at each census was represented by the mean height from the 10 quadrats. Significant changes in vegetation height during the study period and in the mean vegetation height at each census were tested among months and years by means of a two-way analysis of variance (ANOVA) in the Generalized Linear Models module of SPSS ver. 11.5J for Windows (SPSS Inc., Chicago, IL, USA).
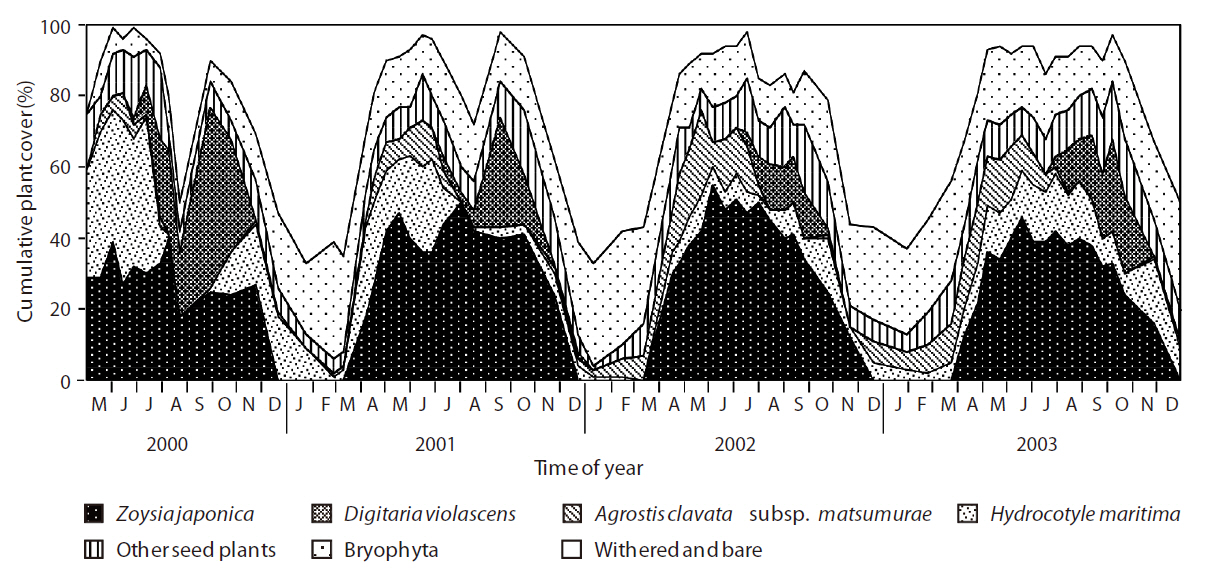
For each species, percent cover was visually evaluated using 11 ranks (from 0% to 100% at 10% intervals). Those species whose mean cover in the 10 quadrats exceeded 20% were defined as dominant species; those with 1-10% cover were defined as species with low cover, and those with < 1% cover were defined as rare species. In the present study, the vegetation structure was represented by a combination of vegetation height and percent cover.
>
Seed phenology and the production of inflorescences on dominant species
The abundance and maturity of the inflorescences of the following three dominant species were evaluated in both the vegetation and sika deer fecal pellets.
Censuses were performed from May 1999 to January 2004;
>
Sampling of sika deer pellets and seeds contained in the pellets
To estimate mean fecal pellet mass, two pellets were collected from each of five pellet groups on each of six sampling occasions between May and August (
Sika deer fecal pellets were collected approximately every 2 weeks from April to July and monthly from August to November from May 1999 through November 2001. Only fresh pellets were collected, after removal of any surface material that might have contaminated them with uningested seeds. Because excretion of seeds by sika deer reaches a peak about 2 days after ingestion (Takatsuki 2006), the time lag between occurrence of seeds in these fresh pellets and seed phenology can be considered negligible. To determine the species composition and density of seeds per pellet, two (in 1999) or four pellets (in 2000 and 2001) were collected from each of five pellet groups on each sampling occasion (a total of 90 pellets in 1999 and 220 pellets each in 2000 and 2001). The pellets were air-dried and maintained at room temperature (20-25°C). Each pellet was soaked in water in a Petri dish until it had absorbed enough water to soften (approximately 1-h), and was then carefully picked apart with tweezers. Seeds were separated from the pellets under a stereomicroscope (40-100× magnification) and then identified to the species level (where possible), according to specimens in a seed herbarium and specimens collected
Species composition, vegetation height, and plant community dynamics
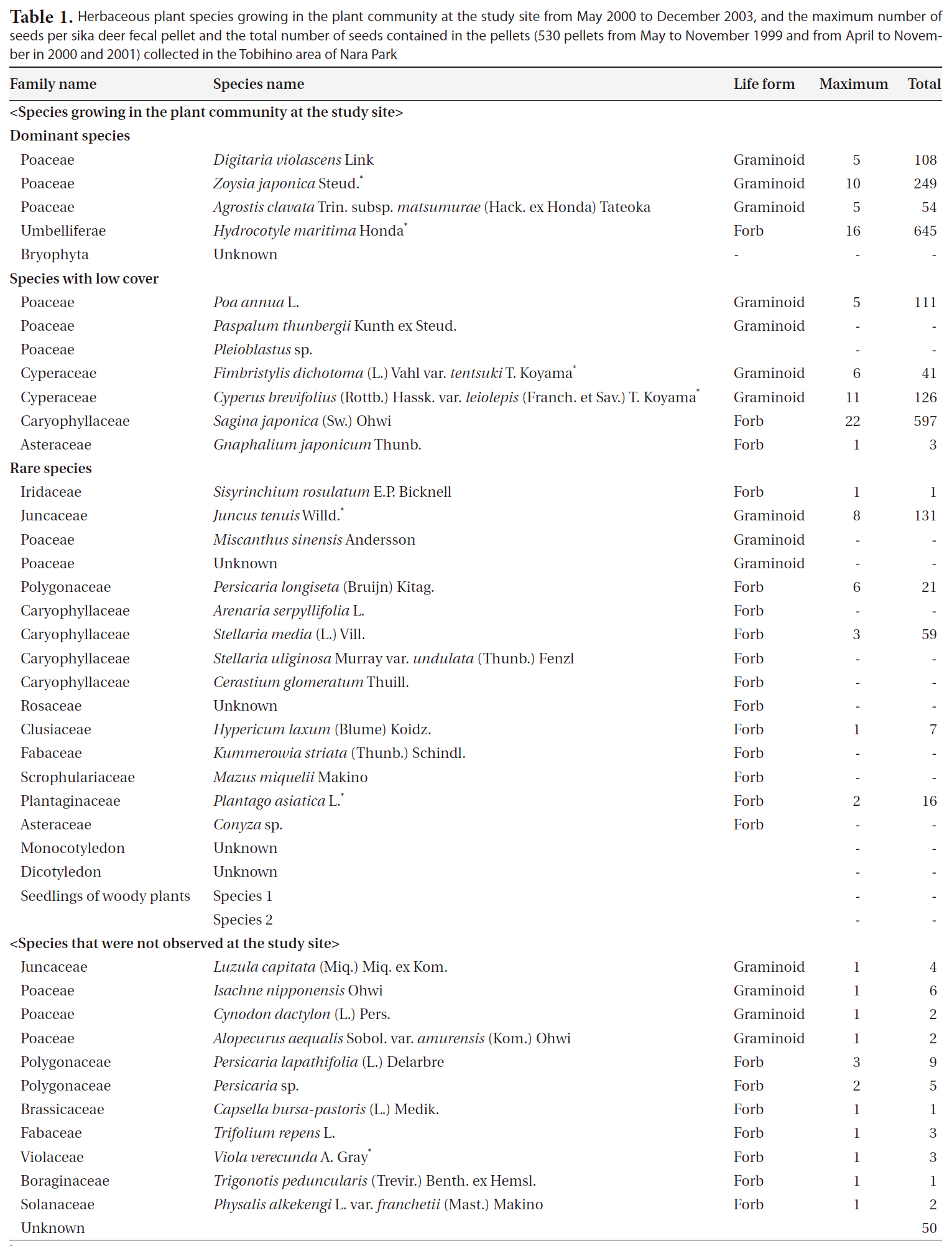
At least 30 plant species were recorded during the study period: 27 species were herbaceous plants belonging to 13 families (10 graminoids, 15 forbs, one grass, and one herb), one species was a bamboo, and two species were woody seedlings (Table 1). Of the 27 species, the
dominant species were
Vegetation height began to increase in March, reached a peak of 100-120 mm in June (the heights of the flowering stems of
The total plant cover also showed a periodic trend (Fig. 2). The cover exceeded 70% from spring to autumn, except for a drop to approximately 50% in August 2000 and declined sharply in winter to less than 20% (excluding bryophytes). The cover of the four dominant species accounted for 60-80% of the plant community during the growing season. Among the dominant species,
showed a less obvious periodic trend among the 4 years, but the trends were similar in 2000 and 2001 and again in 2002 and 2003.
>
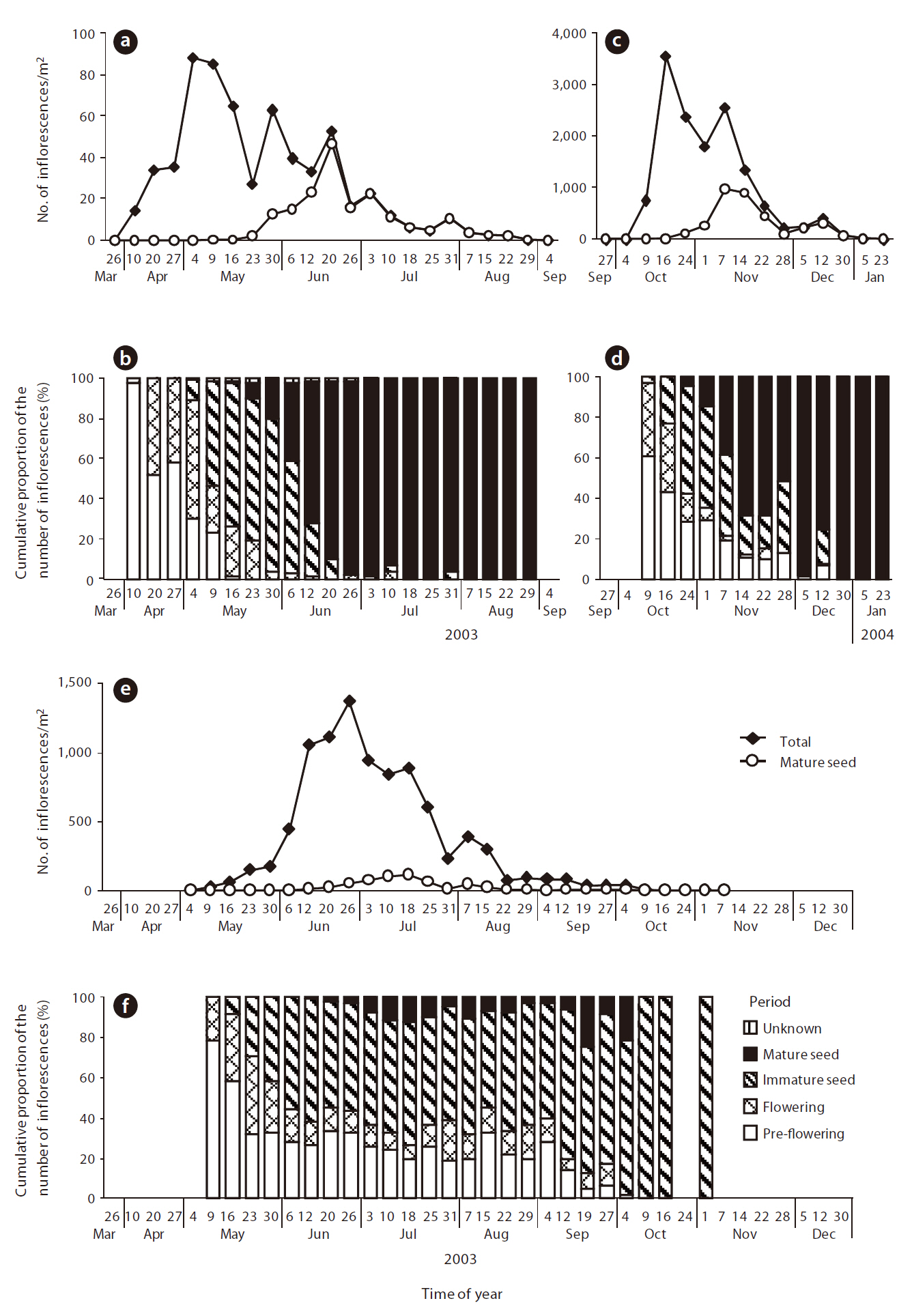
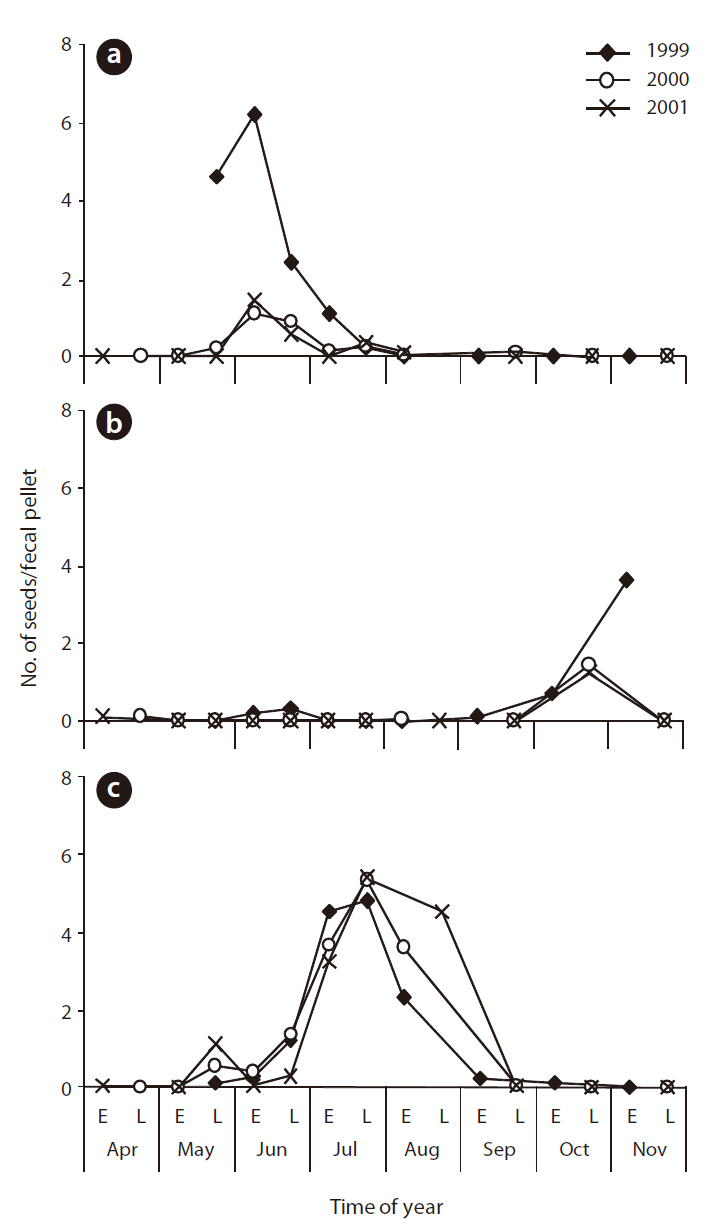
Seed phenology and production of Z. japonica, D. violascens, and H. maritima inflorescences
The maximum number of
Because of the similarity among phenologies, only
>
Seeds in sika deer fecal pellets
The mean number of pellets per defecation was 60.4 ± 14.1 (mean ± standard deviation,
In total, 530 pellets (approximately 100 g dry weight) from 155 fecal pellet groups were collected during the study. Overall, 2,257 seeds belonging to at least 26 species (11 graminoids and 15 forbs) from 15 families were extracted from the pellets (Table 1). Of the 26 species,
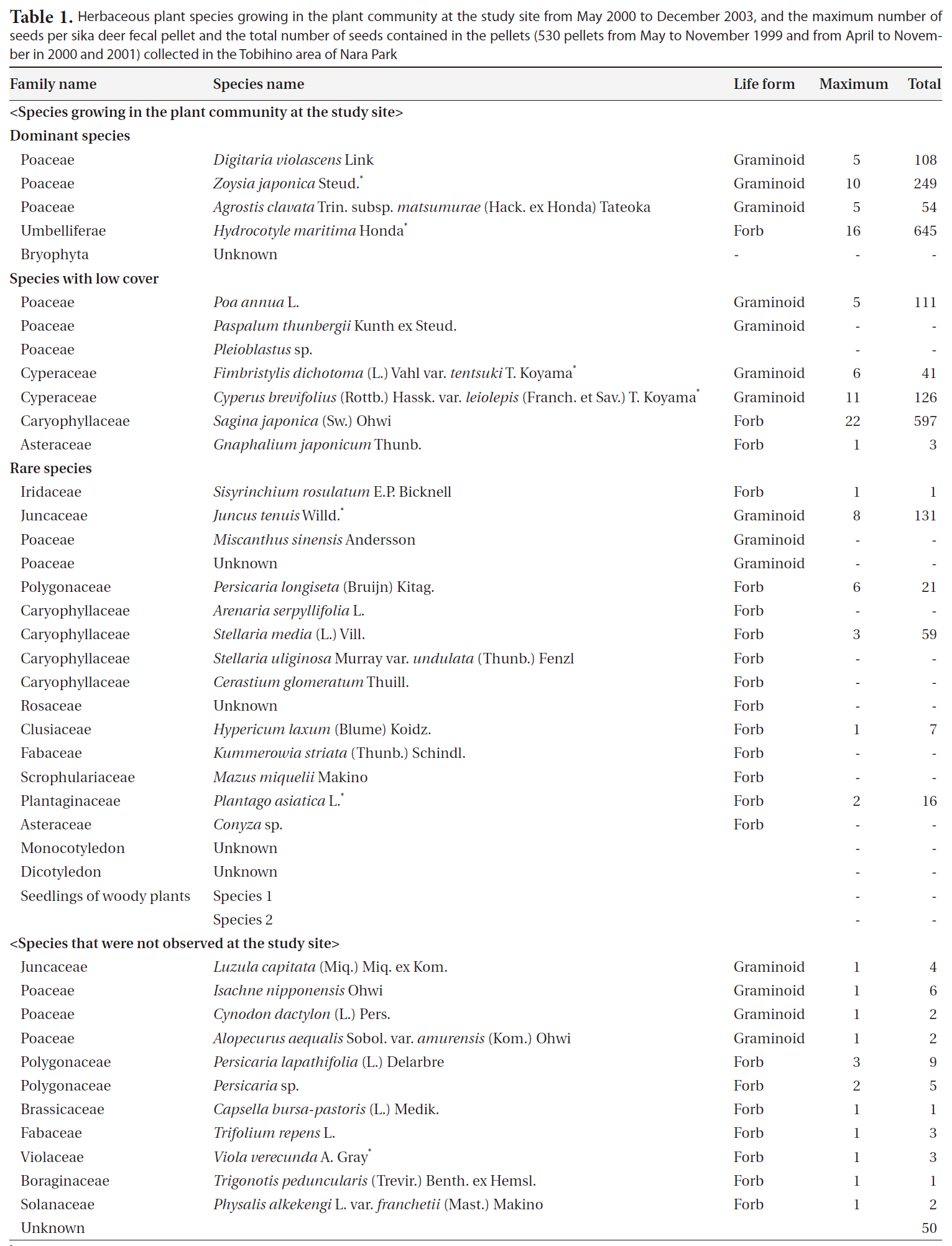
Herbaceous plant species growing in the plant community at the study site from May 2000 to December 2003 and the maximum number of seeds per sika deer fecal pellet and the total number of seeds contained in the pellets (530 pellets from May to November 1999 and from April to November in 2000 and 2001) collected in the Tobihino area of Nara Park
58% (15 species) corresponded to species previously observed at this site. The total number of seeds from these 15 species accounted for 96.1% of all the seeds in pellets. The maximum number of seeds per pellet was 29 in May 2001. Of the identified species, no intact woody-plant seeds were present. The seeds of
The abundance of seeds extracted from the pellets differed among herbaceous species, but could be roughly classified into three groups according to the period of seed occurrence: spring-summer, midsummer, and autumn. When analyzing the species with a monthly mean of more than three seeds per pellet during the study period,
>
Vegetation structure and dynamics of the herbaceous plant community
The vegetation of the Japanese archipelago, which mostly experiences a warm-humid temperate climate, is dominated by forests, and most of the existing grasslands have been established under conditions of ongoing physical disturbance or stress, such as mowing or grazing (Tagawa 1973). Under heavy intense grazing by large mammals, the vegetation of such grasslands develops as a community dominated by
Obvious declines in vegetation cover of all species occurred in August 2000 due to partial stripping off of the herbaceous vegetation layers by wild boars in late July and an unusually low amount of rain in August (only 18 mm). As a result of the low precipitation, the vegetation’s ability to produce new biomass decreased. Although part of the area that was disturbed seriously by the boars was invaded by bryophyte species, most of the vegetation at the study site recovered after this combination of biotic and abiotic disturbances.
>
Seed contents of sika deer pellets
Seeds of at least 26 herbaceous species were extracted from sika deer pellets. In the same study area, seedlings of nine of the 26 species (five graminoids and four forbs) and an additional four forb species (
The species composition of seeds contained in fecal pellets generally reflected the vegetation at the study site; at least 58% of all species (15 of 26 species) at the study site were contained in sika deer fecal pellets, and the total number of seeds of the 15 species accounted for 96.1% of all seeds in the pellets. On a subtropical island in southern Japan, graminoid seeds dominate sika deer pellets, and more than 90% of them belonged to Cyperaceae, which are dominant in one of the foraging areas on this island (Yamashiro and Yamashiro 2006). These results reflect correlations between the abundance of the species in large herbivore dung and that in the foraging area (Couvreur et al. 2005). Although the number of seeds collected was very small (38 of 2,257 seeds), 42% of all species (11 of 26) in pellets could not be observed growing at the study site. Thus, sika deer also graze in other parts or outside of Nara Park and imported seeds from those areas to the study site.
The seed contents of pellets tended to be abundant for the dominant plant species in the community; many seeds of the dominant species
>
Comparison between seed phenology and seeds in pellets
In Nara Park,
Based on the results of seed production and seed abundance in pellets for
Previous studies on seed dispersal by large herbivores (Malo and Suarez 1995, Pakeman et al. 2002, Cosyns and Hoffmann 2005, Couvreur et al. 2005) have not considered the loss of immature reproductive organs caused by grazing. The comparison between seed phenology in the community and seeds in sika deer pellets data in this study suggests that numerous reproductive organs may be consumed at immature stages by grazing deer, and that germable seeds excreted by the deer may be a slight part of seed production in the plant community. When fertile