Wetlands provide various habitats for organisms and are ecologically beneficial for mitigating floods, water pollution, and global warming by storing nitrogen and carbon dioxide (Mitsch and Gosselink 2000). Thus, wetlands are considered one of the most valuable ecosystems on Earth (Lee 2000). As recognition for their ecological value has grown, wetland restoration is being conducted Wetlands provide various habitats for organisms and are ecologically beneficial for mitigating floods, water pollution, and global warming by storing nitrogen and carbon dioxide (Mitsch and Gosselink 2000). Thus, wetlands are considered one of the most valuable ecosystems on Earth (Lee 2000). As recognition for their ecological value has grown, wetland restoration is being conducted actively. In particular, abandoned paddy fields are useful as wetlands in an ecological context, because rice fields themselves are one of the most important wetlands and are widely distributed in Korea.
Although total farmland area has sharply decreased since 1995, the area of abandoned paddy fields has increased(Ministry of Agriculture and Forestry in Korea mea2004), and abandoned paddy fields face secondary succession. Abandoned paddy fields have high plant diversity and contain various habitats for fish, amphibians, and reptiles. They also perform an aesthetic function; thus, becoming recognized as important wetlands (Koo 2003). Abandoned paddy fields at the early successional stage serve as habitat for Nannopya pygmaea, an endangered dragonfly species in Korea (Yoon et al. 2010). Juncus effusus (soft rush or common rush) is the predominant species in fallow rice fields abandoned for 3-10 years (Lee et al. 2002) and plays an important role in N. pygmaea habitats (Yoon et al. 2010). Therefore, it is necessary to understand the environmental characteristics of J. effusus to restore, create, or manage N. pygmaea habitats.
Additionally, J. effusus is an ecologically important plant that is common in fresh and brackish water habitats (Godfrey and Wooten 1979) and is widely used in water gardens and for mitigation in constructed wetlands.
Juncus effusus has been well studied with respect to its autecology (Richards and Clapham 1941a, 1941b, Lazenby 1955a, 1955b, Grime et al. 1990, Ervin and Wetzel 1997, Wetzel and Howe 1999), potential interactions with other wetland plant species (Ervin and Wetzel 2000, 2001), and the distribution of plants occurring during succession (Comin et al. 2001, Lee et al. 2002, Koo 2003). Numerous studies have indicated that J. effusus is a prominent component of soil seed banks (Chippindale and Milton 1934, Champness and Morris 1948, Thompson and Grime 1979, Grime et al. 1990). The taxonomic characteristics (Lee 2003, Oh and Lee 2003) and distribution of J. effusus (Kim and Ko 1980) have been studied in Korea.
Despite its ecological importance, usefulness, and previous J. effusus research results, information about its environmental requirements is insufficient for restoration and management. The lack of information about the optimal inhabiting environments for wetland plants obstructs successful wetlands restoration and management (Kim and Cho 1999, Kim 2003). The J. effusus environment must be investigated during a dry risk period (May-June in Korea), because this plant requires high moisture for germination and growth, to understand its optimal settlement pattern and the growth environment. The objective of this study was to identify the biotic and abiotic factors that comprise the habitat of J. effusus. These results will provide basic data for introducing J. effusus to created or restored wetlands to provide habitat for the endangered species N. pygmaea.
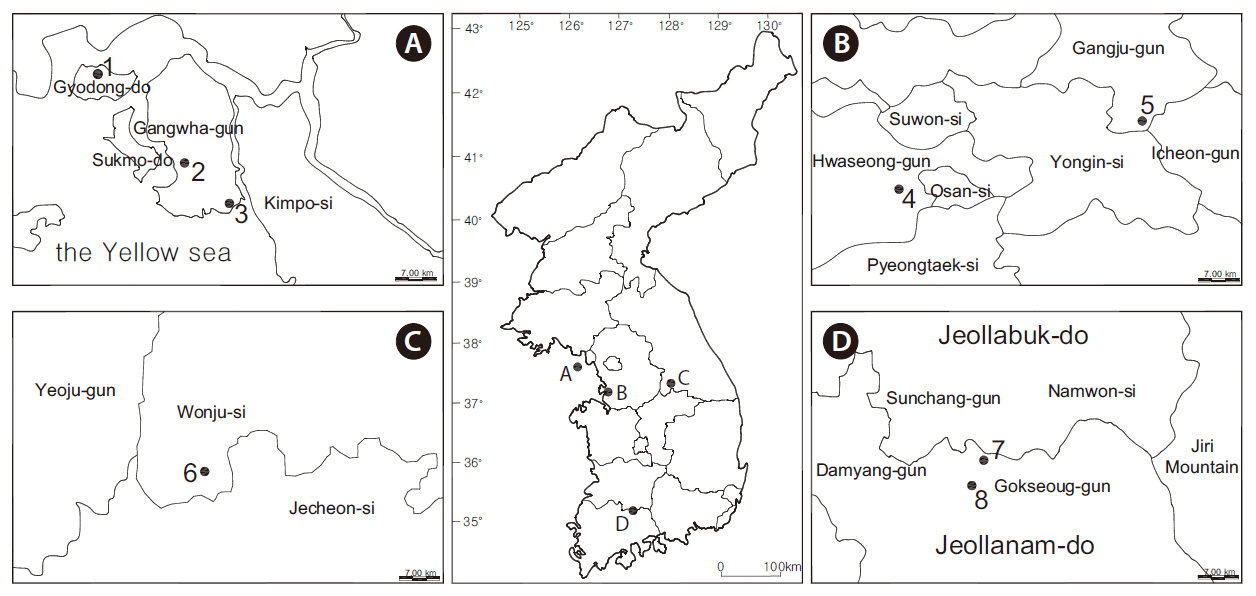
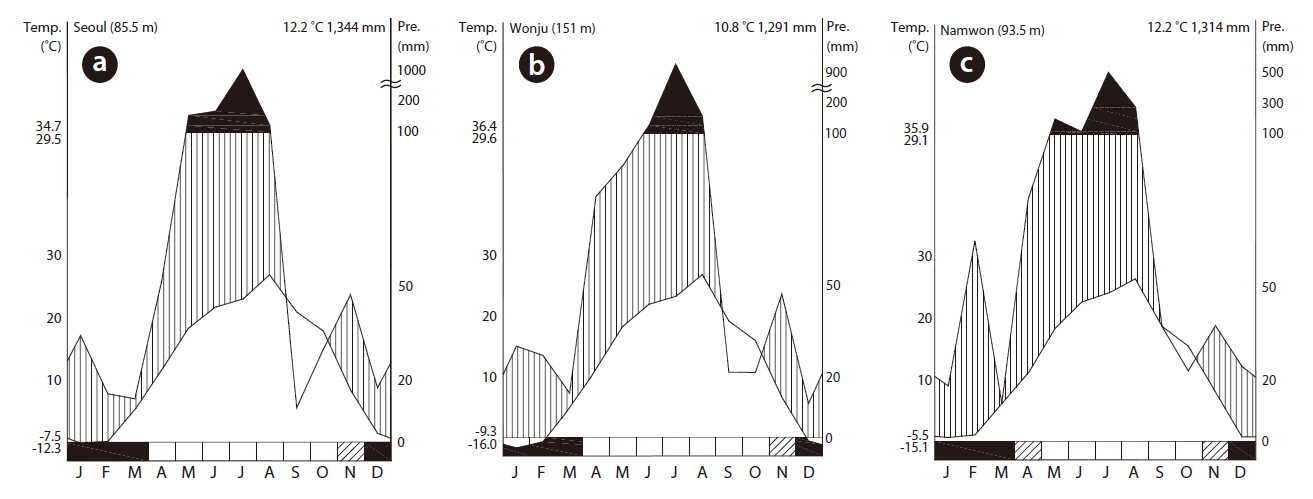
Eight wetlands in South Korea that were dominated by rush were selected (Fig. 1). The average annual temperatures were 13.0, 12.2, and 12.8°C for Seoul (Fig. 2a), Wonju (Fig. 2b), and Namwon (Fig. 2c), respectively, whereas annual precipitation was 140.2, 130.0, and 115.1 mm, respectively (Fig. 2). Most of the studied wetlands were abandoned paddy fields aged 3-10 years. Rainfall and ground water were the main water sources at the study sites. The highest water level was set at 20-30 cm by small banks during the wet season.
We studied vegetation during the driest growing period in Korea (May 27-June 16, 2006). We established 63 randomly distributed 1 m × 1 m quadrats in J. effusus-dominated areas of the study sites. We measured cover, abundance, and height according to a modification of the plant sociological method of Braun-Blanquet (Mueller-Dombois and Ellenberg 2003, Kim et al. 2004). Plant species were identified following Lee (2003) and Lee (2006). Additionally, we investigated the number, width, and height of the tussocks formed by J. effusus per square meter.
Water samples were collected at each quadrat. Water depth (WD) was defined as the distance from the water table to the soil surface; it was positive when the soil surface was below the water table and negative when the soil surface was above the water table. Water temperature and turbidity (TUR) were measured with a thermocouple thermometer (Center 300; Center Technology Co., Shu-Lin, Taiwan) and turbidimeter (Hach 2110P; Hach Co., Loveland, CO, USA), respectively. Dissolved oxygen, conductivity (CON), and total dissolved solid (TDS) were measured using a Corning Checkmate II (model 311; Corning, Lowell, MA, USA), and pH was measured using a pH meter (model AP 63; Fisher, Pittsburgh, PA, USA) in the field. Water samples were collected at each site and filtered with a membrane filter (pore size, 0.45 ㎛). The nutrients in the water samples were analyzed in the laboratory. NO3-N, NH4-N, and PO4-P were analyzed by the hydrazine method (Kamphake et al. 1967), indo-phenol method (Murphy and Riley 1962), and ascorbic acid reduction method (Solorzano 1969), respectively. Cation contents such as K+, Ca2+, Na+, and Mg2+ were mea-
sured with an atomic absorption spectrometer (model AA240FS; Varianm Palo Alto, CA, USA).
Soil was collected at a depth of 0-5 cm from the surface at each quadrat using a soil hand auger. Gravel and large organic debris were removed from the samples, and they were passed through a 2 mm sive (standard sieve #10). Soil texture was determined using the hydrometer analysis method and a texture triangle (Sheldrick and Wang 1993). Organic matter content was analyzed by the loss-on ignition method (LOI) (Boyle 2004). Soil moisture was measured as fresh water moisture, which considers the total amount of water in the sample and as air-dry moisture, and reveals soil particle moisture in the sample (Topp 1993).
Soil solutions were prepared by mixing the soil samples with distilled water at a mass ratio of 1 to 5, and pH, CON, and TDS were measured. NO3-N and NH4-N were extracted with 2 M KCI solutions (Km et al. 2004) and measured colorimetrically using the hydrazine and in dophenol methods, respectively (Murphy and Riley 1962, Kamphake et al. 1967). PO4-P was extracted with Bray No. 1 solution (Bray and Kurtz 1945) and measured colorimetrically using the ascorbic acid reduction method (Solorzano 1969). Total carbon (T-C), total hydrogen (T-H), and total nitrogen (T-N) were determined with an elemental analyzer (model EA1110; CE Instruments, Wigan,UK) at th National Center for Inter-University Research Facilities, Seoul National University. K+, Ca2+, Na+, and Mg2+ were extracted with N ammonium acetate solution (Allen et al. 1974) and measured with an atomic absorption spectrometer (model AA240FS; Varian).
We calculated importance values (I.V.), occurrence frequency (Occ.), and the diversity index (H’) for the vegetation data in each quadrat (Shannon and Weaver 1949, Kim et al. 2004):

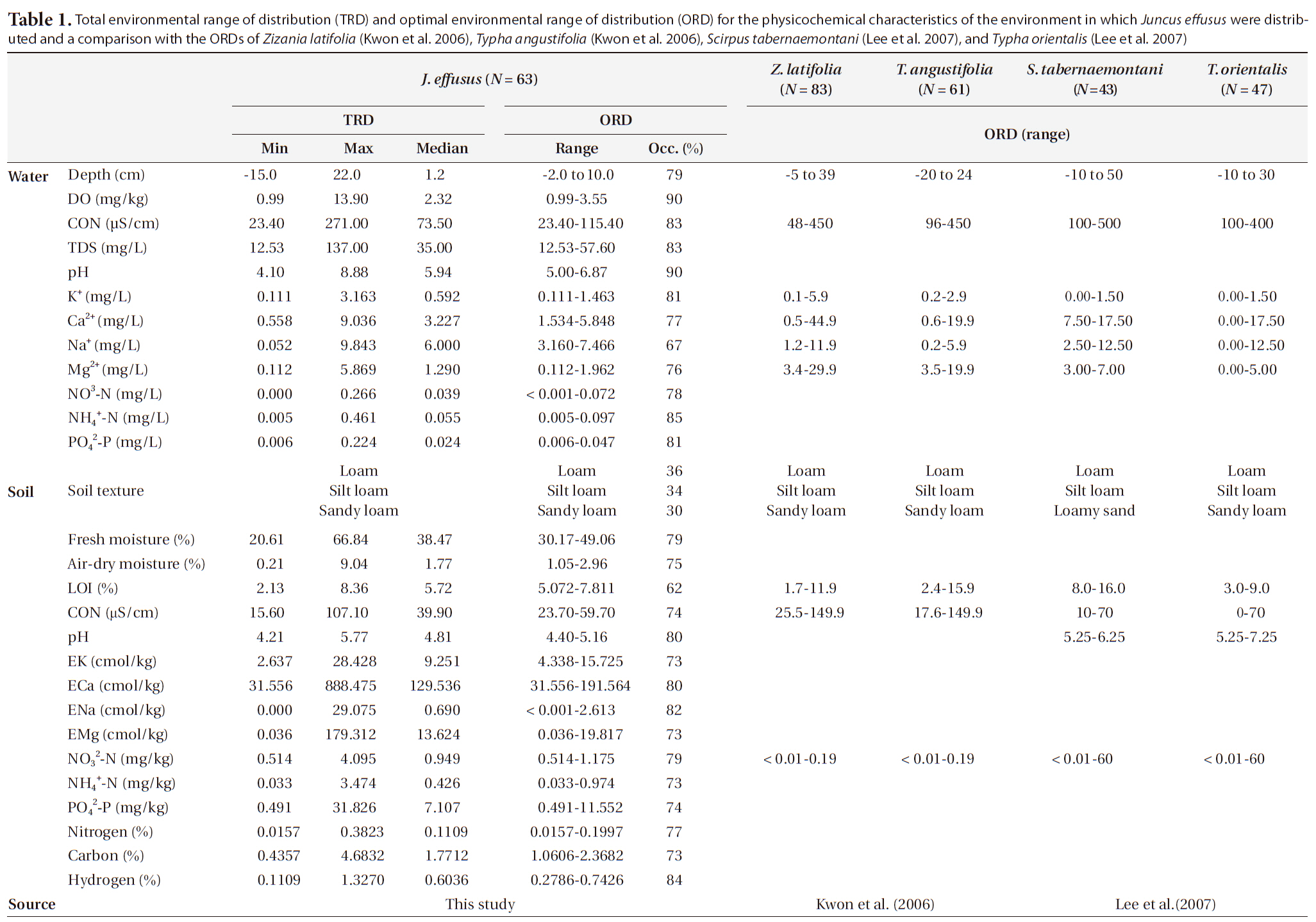
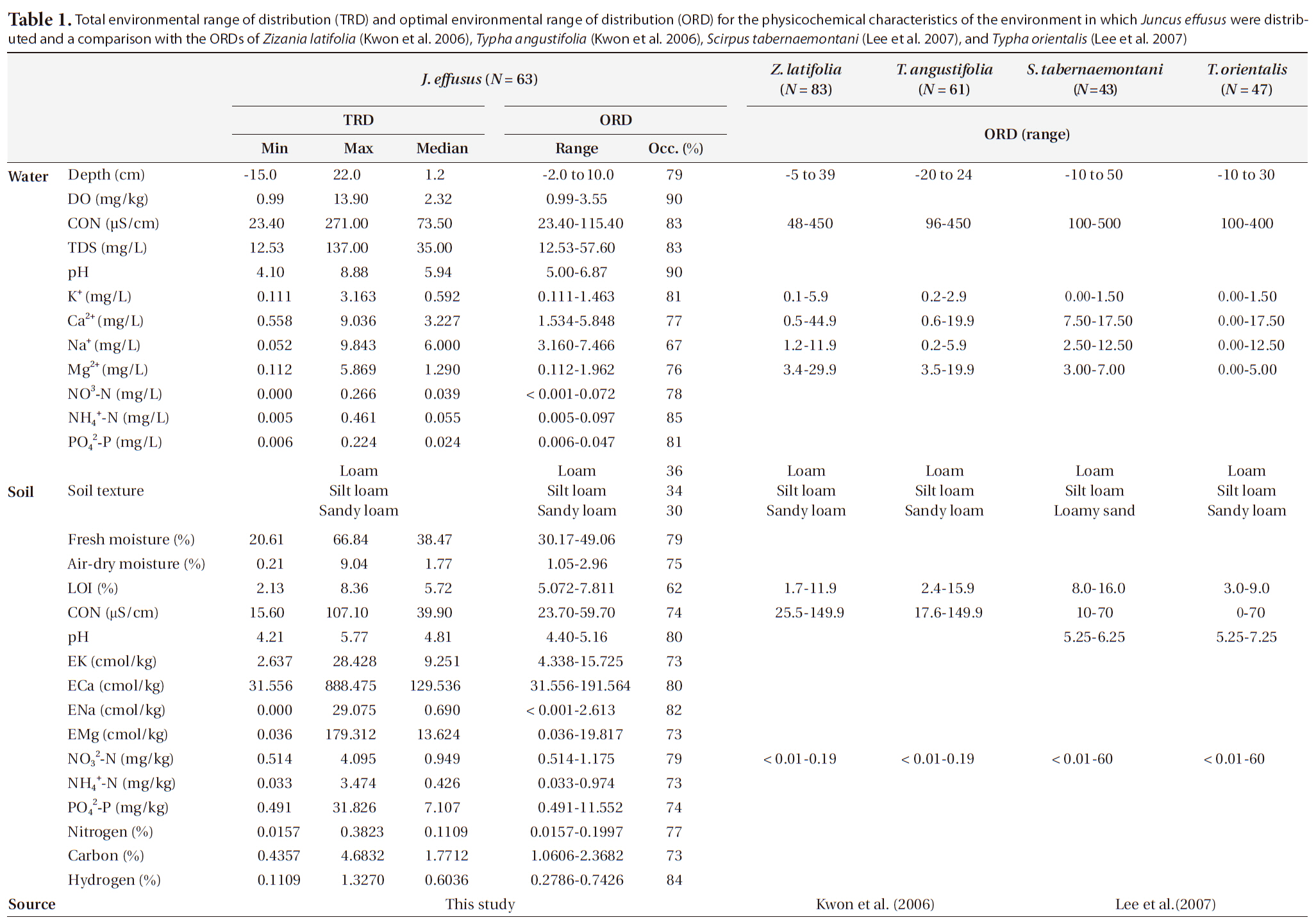
To determine the optimal environment for the growth and distribution of J. effusus, we divided each environmental variable into several classes using Sturges’ rule (Sturges 1926). We obtained distribution patterns and categorized them into several classes according to the environmental variables shown in Figs. 3 and 4. The results revealed the median, maximum, and minimum values for the I.V., density, coverage, height, and H’ for each range. We defined the optimal range of distribution (ORD) for a plant as the range in which each plant was crowded (Lee et al. 2005, Kwon et al. 2006). ORD is associated with: 1) Occ. 60% ≤ ORD ≤ Occ. 90% and 2) ORD ≤ (maximum value/minimum value). The ORD for each plant included a range narrower than half of the full range of incidence for each environmental variable. The total environment range of distribution (TRD) indicated the tolerance of J. effusus within a range of environmental conditions.
Fifty-three plant species from 28 families were found in the J. effusus community. Persicaria thunbergii co-occurred with J. effusus most frequently and was found in 63.5% of the quadrats. Other species frequently found in this community included Glycine soja (wild soybean), Gramineae species, Oenanthe javanica (water dropwort), and Bidens frondosa (Beggar ticks) with frequencies of 36.51%, 31.75%, 23.81%, and 20.63%, respectively. Plant species that co-occurred at < 20% were Typha orientalis (19.05%), Equisetum arvense (14.29%), Artemisia princeps var. orientalis (14.29%), Phragmites communis (9.52%), Salix koreensis (9.52%), Sium suave (7.94%), Scutellaria dependens (6.35%), Carex maximowiczii (6.35%), Typha angustata (6.35%), Alopecurus aequalis var. amurensis (6.35%), and Erigeron annuus (6.35%). The total number of tussock mounds, which were observed in all investigated quadrats, was 196. The average number of tussocks per 1 × 1 m quadrat was 11. When J. effusus cover was 100%, the diameter of tussock was between 15-40 cm, and the height ranged from 100 to 130 cm in the ORD quadrats. Tussock formation by J. effusus facilitated habitation by other plants; thus, increasing species diversity (Ervin 2007).
Water characteristics
The water regime is a major determinant of plant community development and patterns in wetlands (Kwon et al. 2007). Flooding primarily determines plant distribution and can affect species richness and diversity (Huston 1994, Ferreira and Stohlgren 1999, Stromberg 2001, Riis and Hawes 2002). Additionally, WD affects light transmission as well as chemical changes in sediment and water TUR; thus, becoming a significant environmental factor in the growth of wetland plants (Grace and Wetzel 1981, Spence 1982).
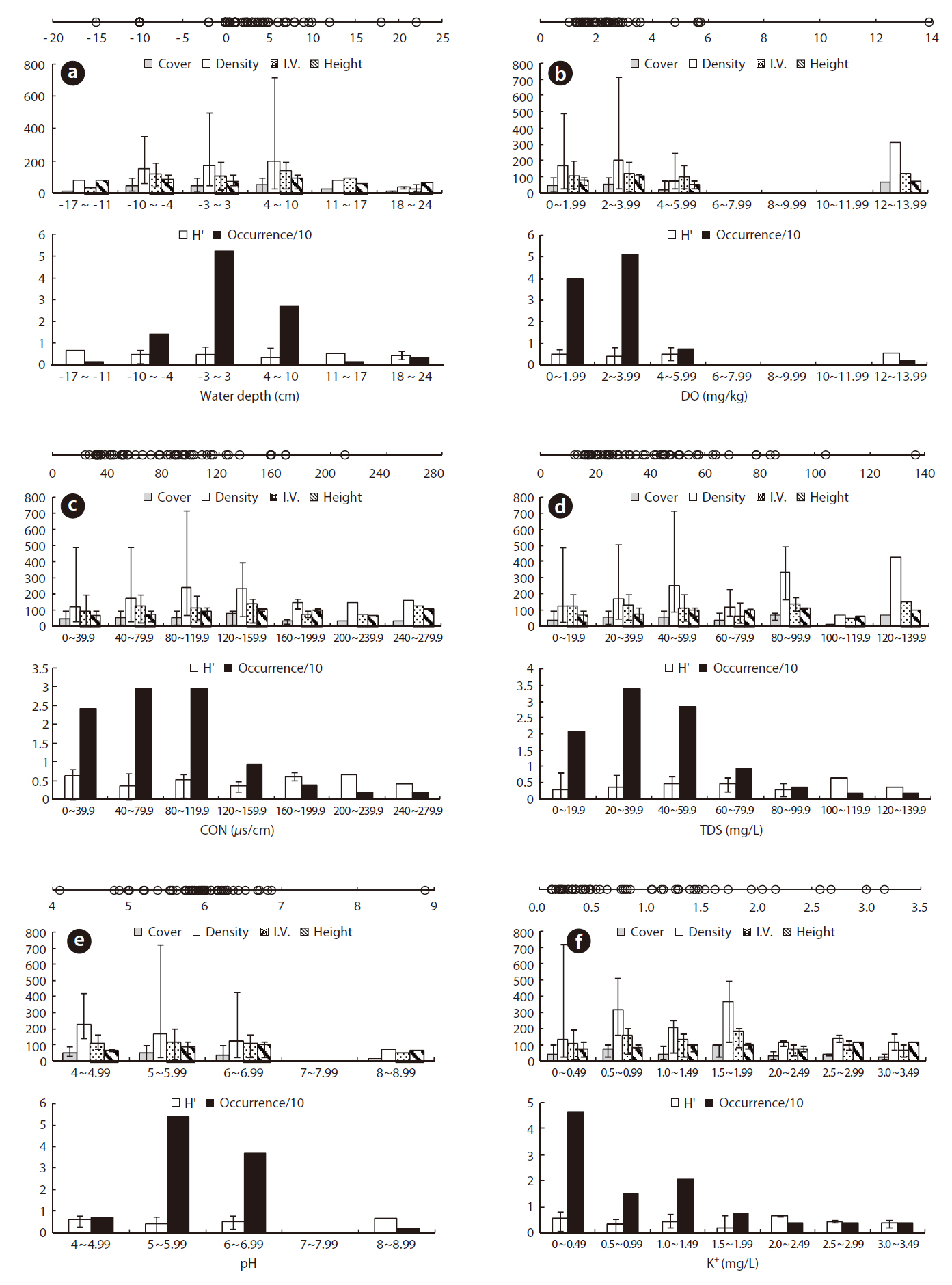
Juncus effusus was growing in a relatively low WD with an ORD of -2 to 10 cm (Table 1) and an Occ. of 79%, which corresponded to the result that J. effusus was well represented in a 3-year-old abandoned field and continued to increase to maximum in a 7-year-old field during the early stage of succession when water had been drained out, i.e., abandoned paddy fields (Lee et al. 2002). In the ORD of WD, the median density, coverage, I.V., height and species diversity of J. effusus was 186 individuals/m2, 52.5%, 116.9%, 92.5 cm, and 0.4, respectively.
The highest water level in which J. effusus was found was 22 cm throughout this research, and most habitats had little flooding and a constant water level. J. effusus is commonly propagated by rhizomes and seeds (Sarma and Rogers 2000). While J. effusus was clearly less abundant on the flooded seed banks and occurred in higher abundances at non-flooded sites, a higher seed density is observed at flooded sites (Gerard et al. 2008). Therefore, the higher seed densities found in flooded sites could not be explained by the presence of large amounts of J. effusus. This contradiction may have been due to hindrance of frequent water level changes on the germination and
growth of J. effusus. Additionally, as J. effusus is a tussock-forming rush, its influence on the distribution and abundance of tussock colonizers differs depending on water level (Ervin 2005). Height and diameter of the tussocks and relative WD are significantly correlated with the distribution of other species (Ervin 2007).
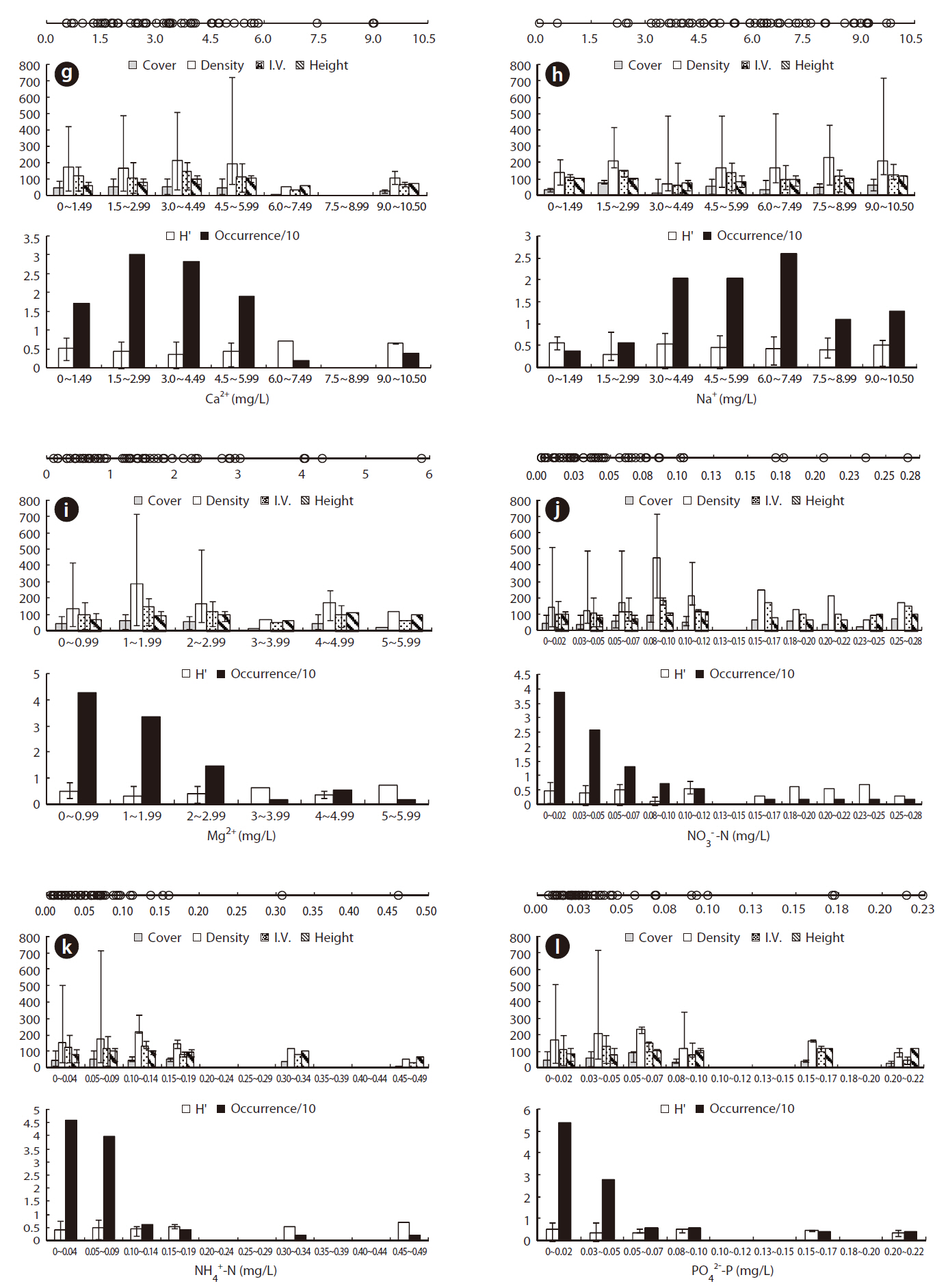
Physicochemical environments in a body of water can influence wetland plants. The ORD of Con, TDS, and cations including K+, Ca2+, Na+, and Mg2+ in J. effusus habitats were lower than those in habitat of other emergent plants, including Scirpus tabernaemontan, Typha latifolia, T. angustifolia, and Zizania latifoia (Table 1). Although J. effusus first appear during the early stages of wetlands when the water is nutrient poor, they seem to survive in oligotrophic environments through the strong uptake of nutrients from the surrounding area. Although the TRD of the water pH was 4.10-8.88, the ORD of the water pH was weakly acidic at 5.00-6.87 (Table 1), with a J. effusus occurrence of 90%.
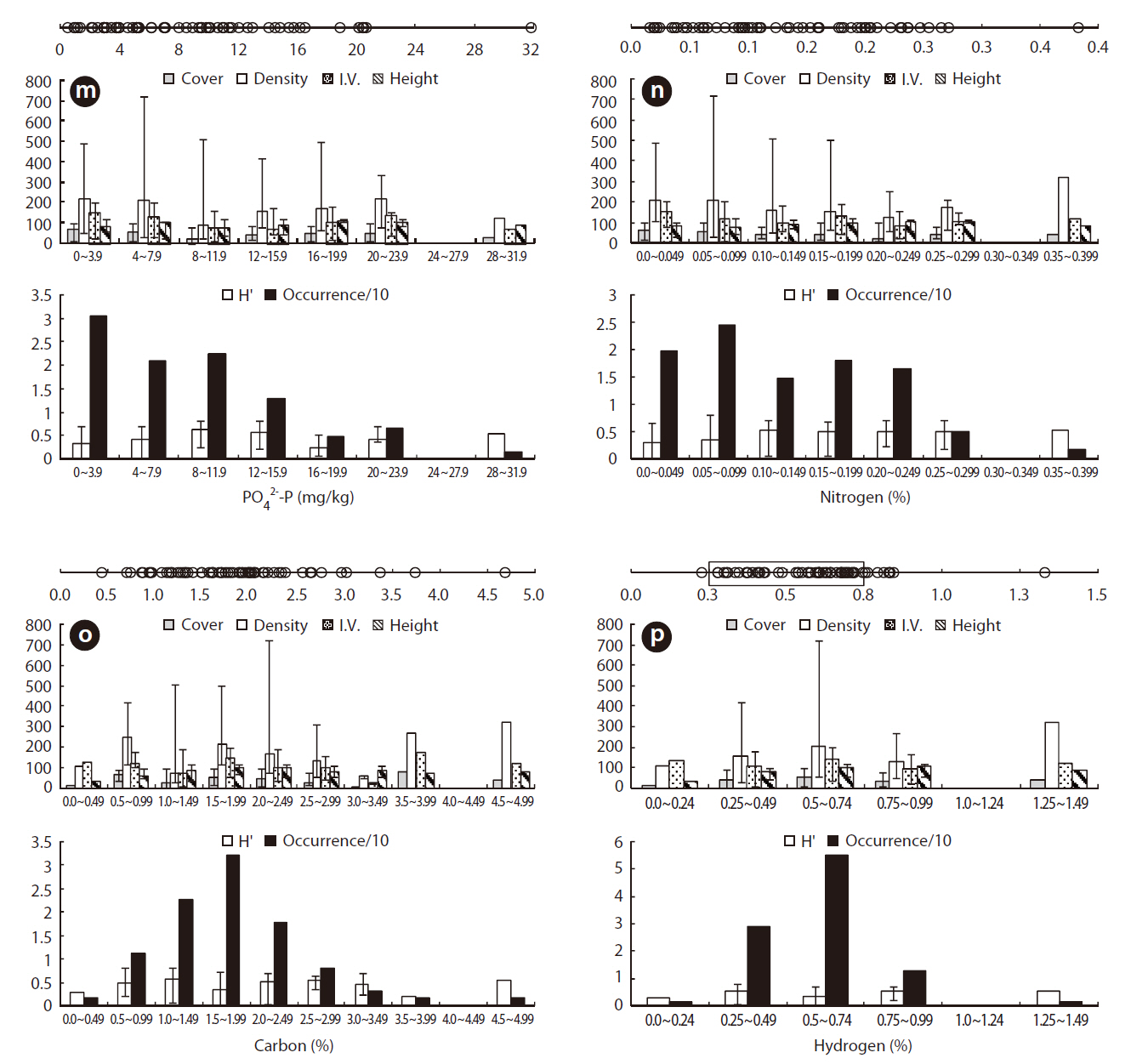
Juncus effusus communities were distributed in habitats with low NO32--N, NH4+-N, and PO42--P. The ORDs for NO32--N, NH4+-N, and PO42--P were: < 0.001-0.072 mg/L with Occ. of 78%, 0.005-0.097 mg/L with Occ. of 85%, and 0.006-0.047 mg/L with Occ. of 81%, respectively.
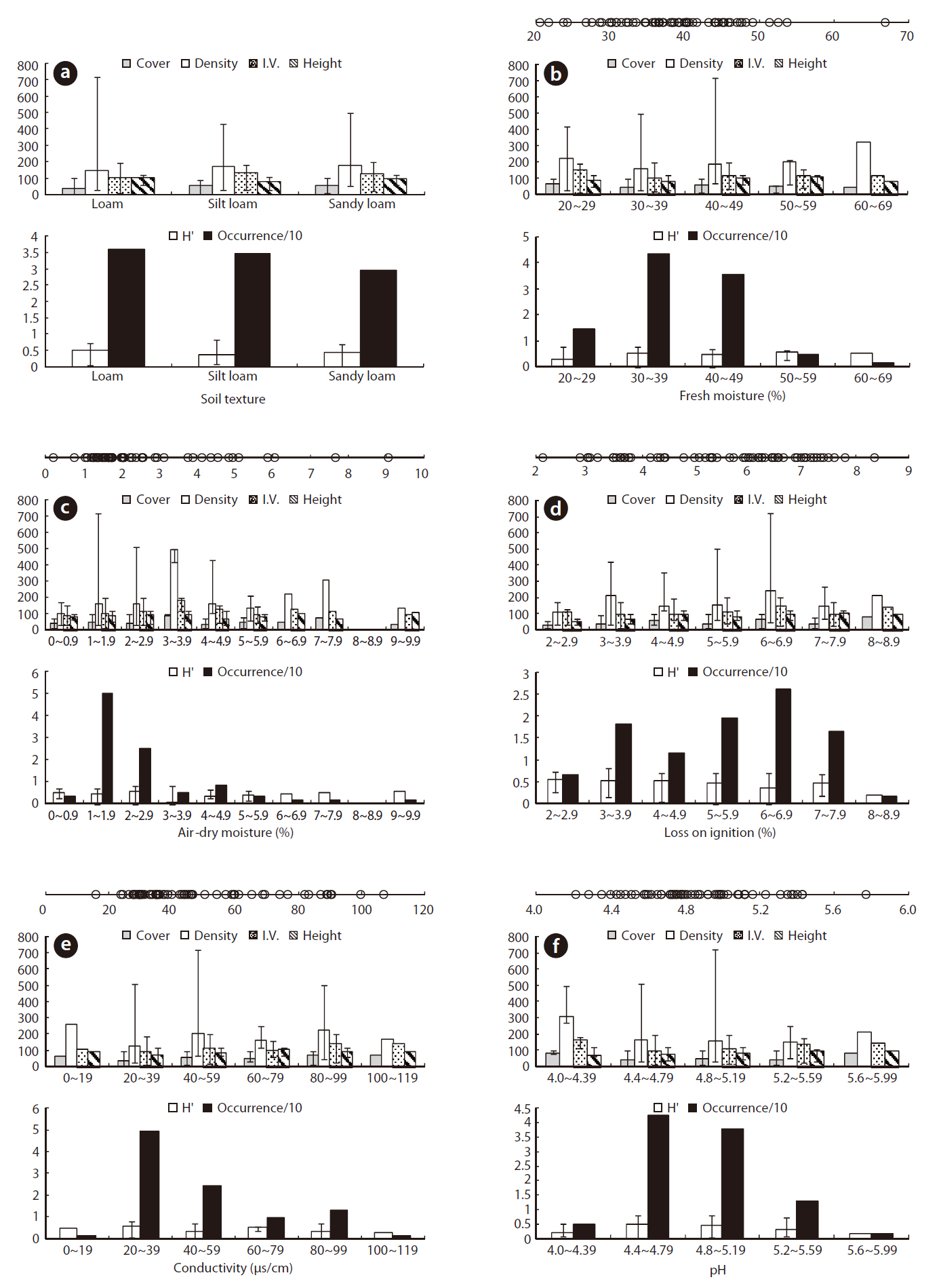
Soil variables are also important when determining plant community composition (Fitter 1982, Keddy 1984, Kwon et al. 2007). Although soil variables may be more important when determining spatial variations in plant community composition and structure, the lack of soil variable data influencing plant distribution makes it almost impossible to evaluate these relationships at present (Kwon et al. 2007). In particular, the vegetation dynamics of many plant communities are thought to be strongly influenced by soil nutrient heterogeneity, but few experimental studies have investigated this relationship. Sixty soil environment characteristics of the TRDs and ORDs of J. effusus are summarized in Table 1, and the J. effusus distribution patterns based on the soil characteristics are shown in Fig. 4.
Soil texture is a major determinant in the distribution and structure of wetland plants (Collins et al. 1987). Absorbing water and nutrients in clay soils is difficult for plants due to the strong adherence of water to clay particles (Robinson 1951, Troeh and Thompson 1993). Besides, high clay content causes high water TUR, obstructing the distribution of submerged plants (Kim 2003). In contrast, high sand content in the planting foundation of emergent plants causes a lack of nutrients, followed by limited growth (Kim 2003). Therefore, determining the appropriate soil texture is key to the growth of wetland plants. The soil texture in the J. effusus habitat implied that suitable soil for growing rush must contain less clay and more silt, and that it is not much affected by its sand content. Plant height and diversity varied considerably in loamy conditions despite fewer individuals (Fig. 3a). Dominance was the highest in silt loam and sandy loams, which had the most individuals.
Soil moisture and organic matter are also significant factors that could determine plant distribution and vegetation structure in wetlands. Organic matter plays an important role in nutrient cycling and retention, because nutrients absorbed by wetland plants can be released by the mineralization and decomposition of organic matter submerged as litter (Mitsch and Gosselink 2000). However, an excess accumulation of organic matter may influence aquatic plant productivity and competition and, thus, moderate organic matter content is important for survival of any aquatic plant (Kwon et al. 2007). In numerous studies, seed density increases with soil moisture due to the presence of high Juncus species seed densities, whose seed survival is favored by anoxic conditions (Thompson and Grime 1979, Bekker et al. 1998). Organic matter in rush habitats is lower than that in S. tabernaemontan habitats (Lee et al. 2005), but the contents were in similar ranges to those of T. latifolia (Lee et al. 2005), T. angustifolia, and Z. latifoia (Kwon et al. 2006) (Table 1). In contrast to these emergent plants, rush accumulate organic matter from the surrounding area by forming tussocks, and habitat nutrient conditions improve with time.
Juncus effusus communities were found in soil pHs of 4.21-5.77. The ORD for pH was 4.40-5.16 with a J. effusus Occ. of 80% (Fig. 4f). Soil pH can also affect plant growth. The acidification of wetland soil can cause eluviation of basic ions and nitrification inactivity, which can restrict plant growth (Marschner 1995). The soil pH ORD for J. effusus was weakly acidic at 5.1-7.8 (Fig. 4f) and represented a broader range of soil pH than the ORD of other wetland plants such as S. tabernaemontan, T. latifolia (Lee et al. 2005), T. angustifolia, and Z. latifoia (Kwon et al. 2006) (Table 1).
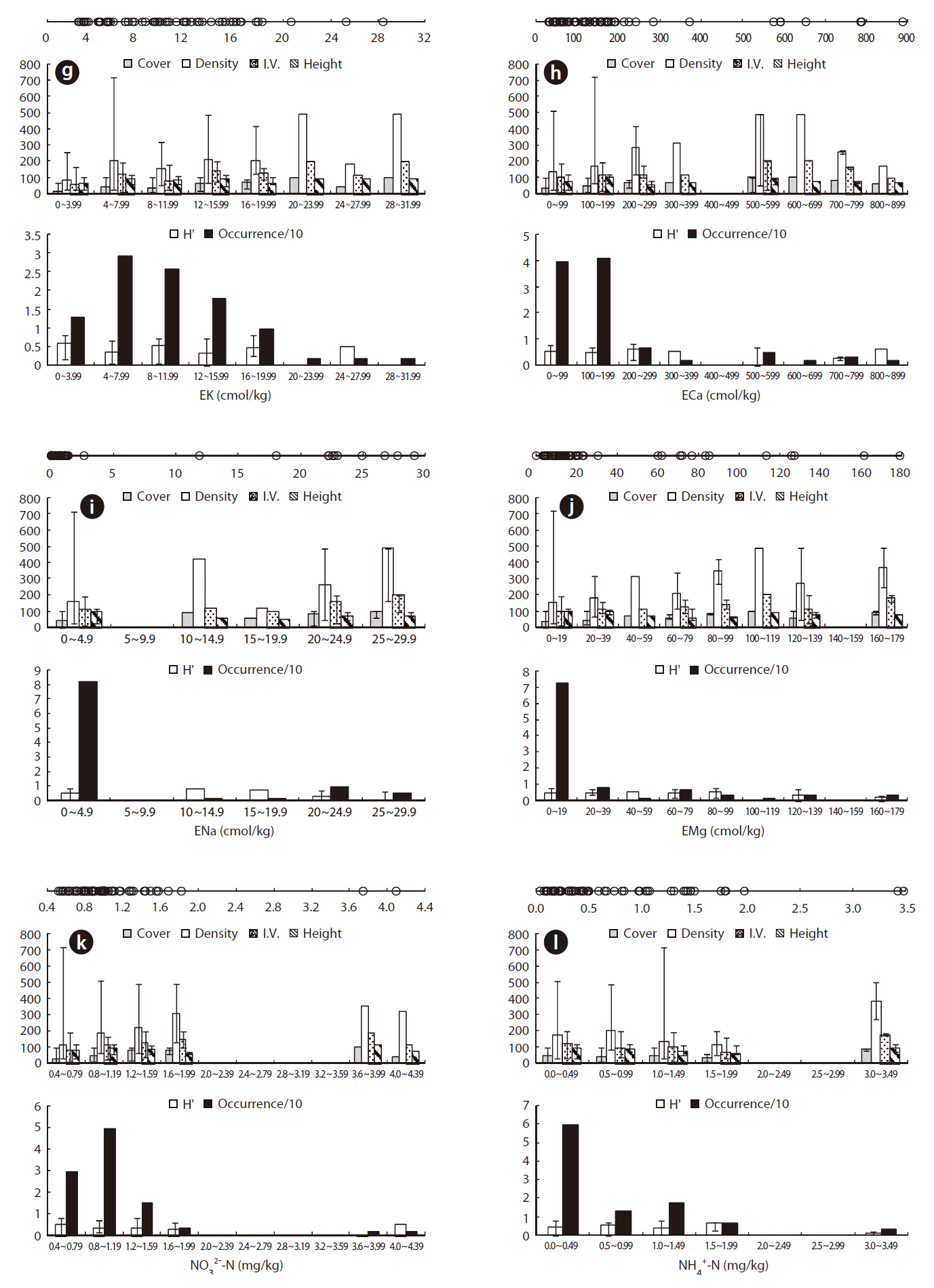
The eK, eCa, eNa, and eMg in the J. effusus communities were 2.637-28.428 cmol/kg, 31.56-888.475 cmol/kg, < 0.001-29.075 cmol/kg, and 0.036-179.312 cmol/kg, respectively. The ORDs of eK, eCa, eNa, and eMg were 4.338-15.725 cmol/kg, 31.556-191.564 cmol/kg, 0.000-2.613 cmol/kg, and 0.036-19.817 cmol/kg, with a J. effusus Occ. of 73%, 80%, 82%, ~ 73%, respectively (Fig. 4g-4j).
Juncus effusus communities were distributed in soils with NO32--N concentrations from 0.514-4.095 mg/kg, NH4+-N concentrations from 0.033-3.474 mg/kg, and PO42--P concentrations from 0.491-31.826 mg/kg. The ORD for NO32--N was 0.514-1.175 mg/kg with a J. effusus Occ. of 79% (Fig. 4k), the ORD for NH4+-N was 0.033-0.974 mg/kg with a J. effusus Occ. of 73% (Fig. 4l), and the ORD for PO42--P was 0.491-11.552 mg/kg with a J. effusus Occ. of 74% (Fig. 4m).
Juncus effusus communities were distributed in soils with T-N concentrations from 0.016-0.382%, T-C concentrations from 0.436-4.683%, and T-H concentrations from 0.111-1.327%. The ORD for T-N was 0.016-0.200% with a J. effusus Occ. of 77% (Fig. 4n), the ORD for T-C was 1.061-2.368% with a J. effusus Occ. of 73% (Fig. 4o), and the ORD for T-H was 0.279-0.743%, with a J. effusus Occ. of 84% (Fig. 4p).
1) The J. effusus habitats harbored G. soja (wild soybean), family Gramineae, O. javanica (water dropwort), and B. frondosa (Beggar ticks) at the highest frequencies, except for P. thunbergii.
2) The optimal WD in the J. effusus habitats ranged from -2 to 10 cm without a sharp fluctuation in water level; thus, abandoned rice fields provide suitable J. effusus habitats. Additionally, the tussocks formed by the rush lead to the introduction of other plants, causing increased species diversity
3) The soil texture ORD included loam, silty loam, and sandy loam. J. effusus growth appeared not to be affected much by sand content.
4) The ORD for fresh moisture was from 30.17% to 49.06%. The ORD for air-dry moisture was 1.05-2.96%, and that for LOI was 5.07-7.81%. Organic matter was relatively lower in content than that for other species, but the introduced J. effusus grew well during early succession despite low nutrition.
5) The water and soil physicochemical environments clearly showed a different distribution than other emergent plants, including S. tabernaemontan, T. latifolia, T. angustifolia, and Z. latifoia. The appearance of J. effusus during early succession indicated relatively a lower proportion of important plant physicochemical parameters, and planting J. effusus may be used to improve sterile conditions. The increase in productivity and organic matter in the habitats after planting will be followed by an increase in nutritive value.