Since the 1960s, the Korean government has promoted petrochemical and heavy chemical industries in Ulsan,Gyeongsangnam-do, and Yeocheon, Jeollanam-do. Along with national industrial growth, associated environmental problems also arose due to increased contaminants from the exhaust of various factories in the industrial complexes. In fact, harmful substances emitted from chemical fertilizer factories damaged crop and forest production in the 1960s. In addition, industrialization also degraded atmospheric conditions due to increased auto exhausts in large cities and continued increase of ozone concentration as a secondary pollutant. Ozone hazard warnings increased from 1995 to 2008, as the frequency rose from only twice (one day) in 1995; 24 times(12 days) in 1997; 52 times (17 days) in 2000; to 101 times(32 days) in 2008. Also, the ozone hazard warning occurred much earlier (April 25) in 2008 than the previous warning announcement on July 22 in 1995. As of 2008, the highest concentration per hour was 203 ppb in Yeosu, Jeollanam-do (Ministry of Environment 2008).
Interest in pollutants and in their effects on living organisms has increased in the last few decades, as a consequence of the rise in their ground-level concentration and of the widening of their diffusion area. Current levels of the pollutant were high enough to exceed the tolerance threshold of many plants, thus impairing plant growth, reducing crop yields, and altering the composition of plant communities. Pollutants could also induce subtle changes at the physiological and biochemical level, which can lead to different plant responses. Fares et al. (2010) reported that stomatal flux, which is considered responsible for ozone damage, showed a weaker correlation with ozone concentrations than non-stomatal flux during the summer and fall seasons. Therefore, much attention has been given toward understand the mechanisms of plant adaptation to pollutants. Ordinarily, high levels of pollutants can delay growth and development, reduce yield, and, in extreme cases, can inflict lethal injuries on plants. To ensure survival, plants have evolved a range of response strategies to various abiotic stresses; these responses vary with genotypes and among specific stresses (Deepak and Agrawal 2001, Azevedo Neto et al. 2006). At the whole plant level, the effect of stress is usually perceived as a decrease in photosynthesis and growth, alteration in carbon and nitrogen metabolism (Cornic and Massacci 1996, Mwanamwenge et al. 1999, Law and Crafts-Brandner 2001, Han et al. 2007), and reactive oxygen production (Turcsanyi et al. 2000, Schwanz and Polle 2001).
However, there have been few studies on injury to trees by oxidative stress under actual field conditions (Vollenweider and Gunthardt-Goerg 2005, Bussotti and Ferretti 2009), especially in terms of physiological damage and defense responses to oxidative stress. In fact, because of the combination of environmental stresses simultaneously occurring in the field, it is difficult to identify the main cause of injuries to tree and natural vegetation under field conditions (Mittler 2006). Since many species exhibit high variability in their symptoms, which could be confused as senescence, such species should not be used in the field as an indicator for ozone injury assessment. Nonetheless, observations of typical symptoms on above-ground plant parts in the field has turned out to be a valuable tool for the assessment of the impact of ambient ozone on sensitive plant species (Skelly et al. 1987, Flagler 1998, Innes et al. 2001). In addition, Bussotti et al. (2003) provided suggestions to improve the reliability of ozone injury assessment on forest plant species by improving the capability of observers to identify and assess ozone symptoms before field surveys are conducted.
Black pine,
In this study, our primary objective was to determine the major reasons that cause differential growth and visible needle injury on the needle of black pine growing in the two industrial areas (Yeocheon and Ulsan) in Yeocheon, South Korea. To accomplish this, we collected and analyzed meteorological and air pollution data, analyzed soil properties, and evaluated physiological characteristics such as photosynthetic pigment content, lipid peroxidation, and antioxidative enzyme activity of black pine from the Yeocheon and Ulsan industrial areas, and their possible relationships.
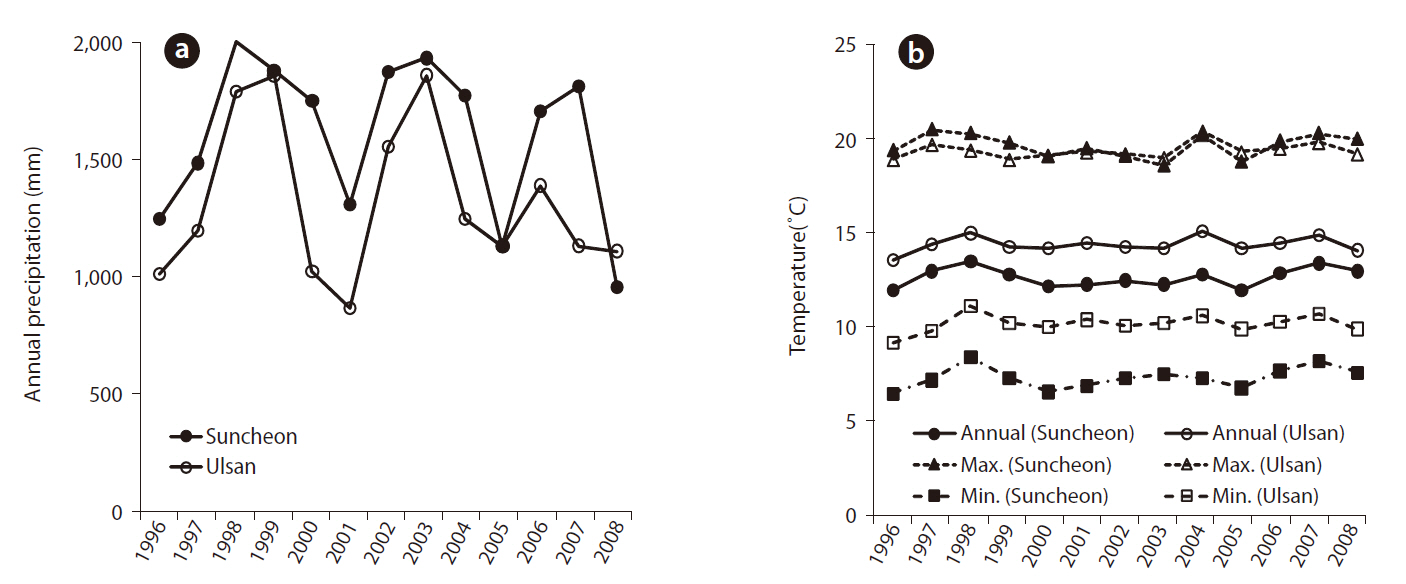
The study was conducted in two experimental black pine forests, which were established in the vicinities of the Ulsan (San 73 Yongyeon-dong, Nam-gu, Ulsan metropolitan city) and Yeocheon (San 100 Wollae-dong, Yeocheon-si, Jeollanam-do) industrial complexes in 1996. These experimental forests were established to examine the tolerance of black pine as candidate trees against environmental pollution. The selection process was based on established selection standards for visual injury, vitality, and physiological responses of black pine trees in response to two environmental conditions typified in the Ulsan (Dotjilsan) and Yeocheon (Wollae) industrial complex. Prior to the establishment of the experimental forest, seeds from thirty candidate black pine trees were sown in Korea Forest Research Institute (KFRI)’s tree nursery for seedling production. Two-year-old seedlings were transplanted to the Yeocheon and Ulsan sites, which consisted of 10 replicates per family laid in three different blocks. Climatic data were collected from the nearest Suncheon and Ulsan observatories for Yeocheon and Ulsan study sites, respectively, and the annual changes in air temperature and precipitation are shown in Fig. 1.
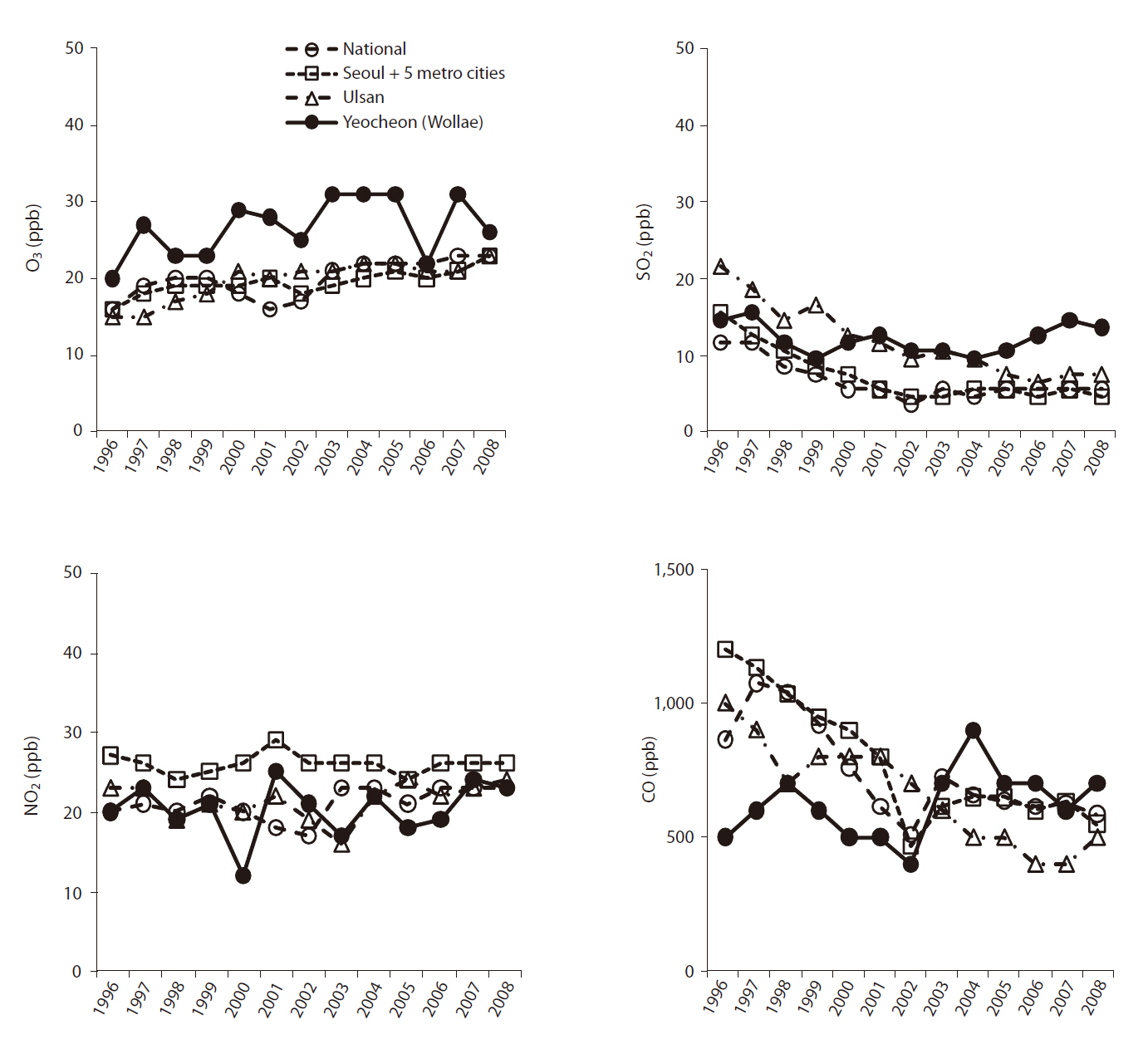
Both study sites are close to either a chemical fertilizer factory or petrochemical and heavy chemical industries. Owing to exhaust gas from the industrial complexes, the trees in two experimental forests were exposed to concentrated air pollutants mainly consisting of such as sulfur oxide (SO2) and ozone (O3). Changes in the annual mean concentration of air pollutants from 1996 to 2008 are shown in Fig. 2.
>
Growth and vitality measurement
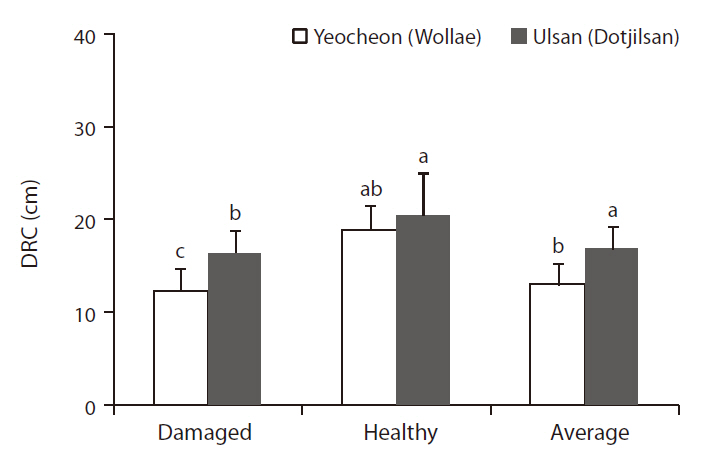
In 2008, after 12 years of field growth, the diameter of root collar (DRC) of the experimental plants was measured at two sites. To assess the adaptation of trees to pollution, bench marking based on tree phenotypes were done, and vitality and visual symptoms were recorded. Tree vitality was assessed based on leaf tip damage, leaf color, and leaf coloring, and each criterion was graded in four levels. The vitality evaluation method is considered to be a fast decision standard to assess the adaptation of trees in polluted areas. On the basis of growth and vitality, 10 trees representing groups of damaged and healthy trees were chosen from two sites. Fig. 3 showed the average DRC of 10 damaged and healthy trees and overall average DRC of both damaged and healthy trees from the two experimental sites.
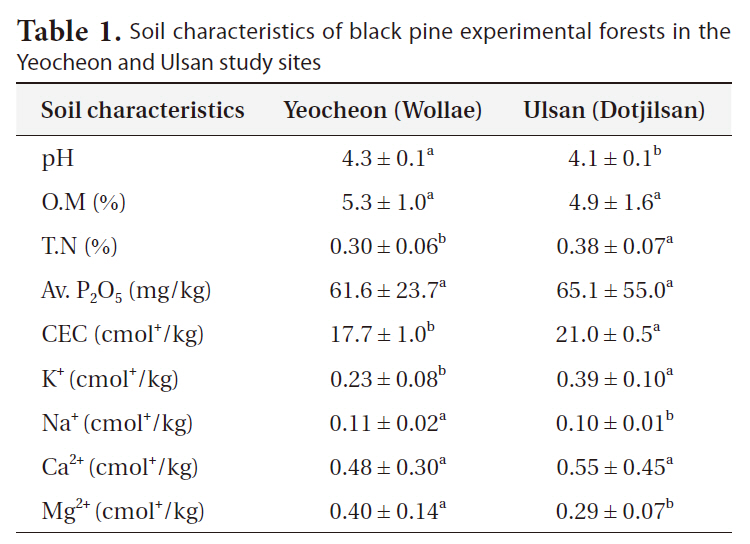
To analyze soil characteristics, 18 soil samples (6 samples per block) were collected from each of the two experimental forests. The mineral soil samples at 20-cm depth were collected using a 3.7-cm diameter core, after removing the organic layer. Samples were taken at four locations approximately 1.5 m from the stem to avoid the impact of stem flow. The samples were immediately processed in the laboratory; roots were removed and passed through a 2-mm sieve. The processed soil samples were refrigerated at 4oC until analysis. Similarly, some subsamples (100 g) were air-dried at room temperature and used for the determination of organic matter content, total nitrogen, available phosphorus, cation exchange capacity (CEC) and exchangeable cations (K+, Na+, Ca2+, and Mg2+). Soil pH (soil:water) was determined from refrigerated samples.
>
Needle sampling and analysis
Needles from the black pines were collected from branch samples located at a height of 2.5-3.0 m from the sun-exposed crowns for both damaged and healthy trees at the Yeocheon sites in August 2008. Since visible injury was not observed in the black needles from the Ulsan site, needle samples were not collected from there.
A total of 10 trees for each group were included in the sampling. Needles from each branch were segregated into current (new leaf) and 1-year-old needles, and refrigerated at 4oC until analysis. The total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid content in the needles were determined based on the procedure described by Lichtenthaler (1987).
Lipid peroxidation was determined by measuring the amount of malondialdehyde (MDA) produced by thiobarbituric acid reaction as described by Heath and Packer (1968). To assess resistance to oxidative stress, the activity of superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), and catalase (CAT) on fresh needles was assayed. SOD was assayed on the basis of the inhibition of reduction of nitro-blue tetrazolium in the presence of xanthine at 530 nm according to the method of Beauchamp and Fridovich (1971). An APX activity assay was carried out in a reaction mixture containing 50 mM phosphate buffer (pH 7.0), 0.5 mM AsA, 0.1 mM EDTA, 0.1 mM H2O2, and 0.1 mL enzyme extract based on the method of Nakano and Asada (1981). The change in A290 was recorded for 1 min after the addition of H2O2. The GR activity assay was carried out in a reaction mixture containing 50 mM phosphate buffer (pH 7.8), 0.1 mM NADPH, 0.5 mM GSSH, and 0.1 mL enzyme extract as described by Carlberg and Mannervik (1985). The change in A340 was recorded for 5 min after the addition of enzyme extract. The CAT activity was determined by following a two-step procedure based on the relationships of dismutation of H2O2 to H2O and molecular oxygen, which is proportional to the concentration of CAT (Fossati et al. 1980). All enzyme activities were measured using a UV-120 (Shimadzu, Kyoto, Japan).
The data were statistically analyzed using Analysis of Variance of a Completely Randomized Design. Means were compared using the Duncan multiple range test at the 5% level. Statistical analyses were done using the SAS System for Windows ver. 8.01 (SAS Institute, Cary, NC, USA).
>
Growth response under two different environments
The two study sites had different growth environments for black pine. As shown in Fig. 1, annual and minimum temperatures in Ulsan were higher than those in Suncheon (Yeocheon site) from 1996 to 2008, whereas the maximum temperature was the same at the two sites. Similarly, the atmospheric concentration of ozone, nitrous oxide, sulfur dioxide, and carbon monoxide, presented in Fig. 2, revealed values of ozone and sulfur dioxide at the Yeocheon site that were much higher than the national average, indicating the occurrence of air pollution in this area. In addition, the total nitrogen, CEC, and K content in the soil of Yeocheon were significantly lower than those of Ulsan (Table 1).
Differences in environmental conditions could lead to differences in black pine growth between the two areas. Based on the DRC of 14-year-old black pines, transplanted at Yeocheon and Ulsan study area in 1996, the average DRC of black pine in Yeocheon was significantly smaller than that in Ulsan (Fig. 3). It is well known that the per-
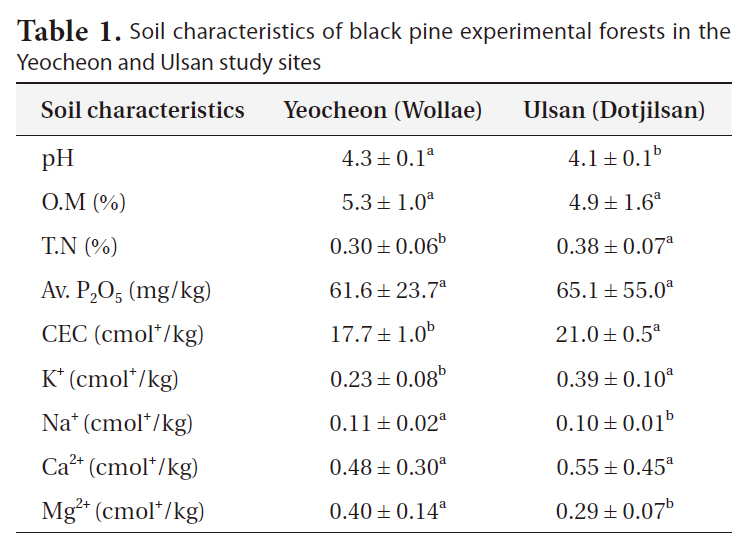
Soil characteristics of black pine experimental forests in the Yeocheon and Ulsan study sites
formance of genotypes relative to each other can vary according to the environment, so that genotypes that are superior in one environment may not be correspondingly superior elsewhere. This phenomenon has been formulated from the concept of genotype-environment interaction (Burdon 1977). In addition, Scholz (1989) described that a particular genotype behaves differently in different environments, and different genotypes vary in their reaction to different environments. In our study, the growth retardation of black pine in Yeocheon might be a result of the mixture of environmental factors, such as lower annual temperature and poor soil conditions, as evident in the lower total nitrogen and CEC of soil as compared with that of Ulsan.
However, chlorotic mottle and tip necrosis, the two most common needle symptoms of conifer species exposed to air pollutants such as O3 or SO2, were observed on the needles of black pines in Yeocheon, but not in Ulsan (data not shown). In general, mottle on young and older needles are induced by low-level exposure to O3, while tip necrosis in young pines is induced by high-level exposures. This means that the lower annual temperature and soil quality may not only be the factor contributing to the growth retardation of black pine at Yeocheon. Our hypothesis is that the growth retardation of black pine at the Yeocheon site could also be due to the reduction of photosynthetic activity due to the presence of necrotic and chlorotic needles. This could be supported by the fact that chlorotic mottle and tip necrosis on black pine needles are induced by oxidative stresses due to pollutants such as O3 and SO2that are prevalent in the Yeocheon area (Fig. 2). As compared to the other sites, the O3 concentration in Yeocheon was highest during the whole period of growth. Likewise, the SO2concentration in most areas decreased gradually from 1996 to 2008, whereas the SO2 concentration in Yeocheon has increased continuously since 1999, and it was the highest among the four areas since 2005. Therefore, it may be that long-term exposure to higher O3 and SO2 concentration have contributed to needle damage and growth reduction of black pine in Yeocheon. Some research has established that ozone, after diffusion through the stomata, causes short-term oxidative stress that can lead to visible injury (Becker et al. 1989, Chappelka and Samuelson 1998, Bungener et al. 1999). Longer-term exposure could eventually reduce shoot and root biomass (Davison and Barnes 1998) or loss of reproductive output (Black et al. 2000). In addition, Hirano and Morimoto (1999) reported that a large reduction in the series of tree-ring index (TRI) in a stand close to an industrial complex appeared from the 1960s to 1970s, and a significant negative correlation between TRI and sulfur dioxide (SO2) concentration was found in the polluted area. Their results indicated that past reduction in the growth of black pine trees in an industrial area was mainly caused by SO2.
>
Individual differences under identical environmental conditions
In general, individual differences in plant growth parameters could be observed in the same environmental conditions. As shown in Fig. 3, in the two study sites there were significant differences in the average DRC of damaged and healthy black pine, as rated according to their viability in terms of such as growth and damage level (data not shown). Interestingly, there was not a significant difference in the DRCs of healthy trees between the two sites, whereas the DRCs of damaged trees showed a significant difference between two sites. This observation is parallel to the findings of Scholz (1989) that different genotypes of the same species differ in sensitivity, and the same dose can lead to different symptom intensities in different individuals of the same species. Comparing
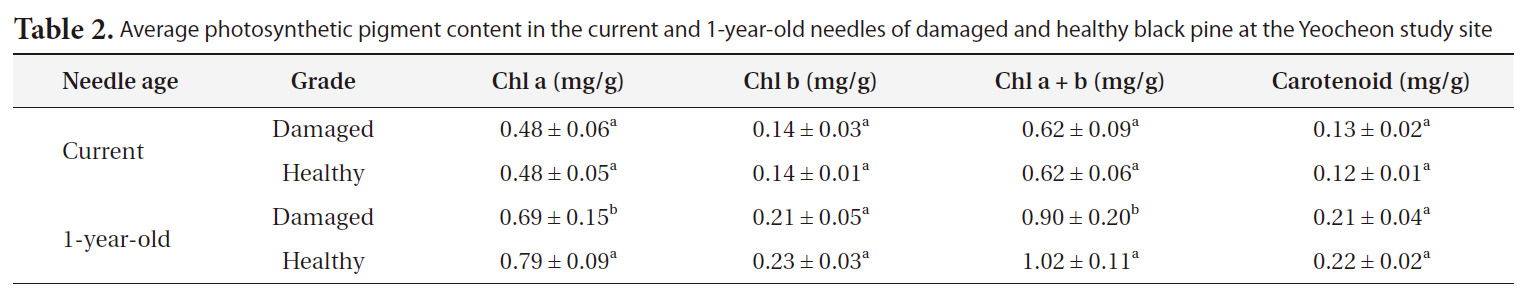
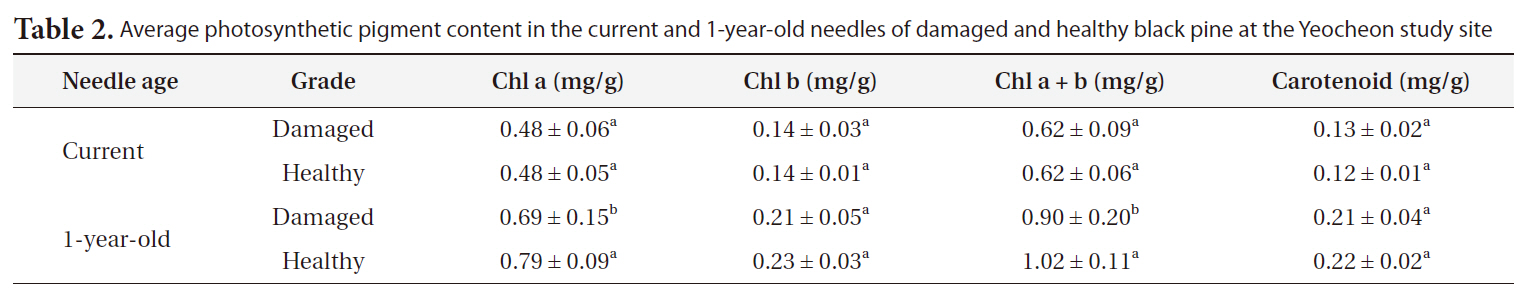
Average photosynthetic pigment content in the current and 1-year-old needles of damaged and healthy black pine at the Yeocheon study site
the two genotypes, it shows that the genotypic variation of sensitivity may be great, and identical genotypes react similarity to a given pollution regime. This means that the phenotypic variation of damage in such experiments was governed to a great extent by genetic factors.
Consequently, in both the Yeocheon and Ulsan experimental sites, the growth and vitality of black pine in differed noticeably enough that we have grouped them into damaged and healthy trees for the purposes of genotypic comparison within the same environmental conditions. However, the difference between the damage and the healthy trees was more obvious at the Yeocheon site, which could be ascribed to the overall environmental conditions characterized by lower total nitrogen and CEC in the soil, lower annual temperature, and higher air pollutants. These poor environmental conditions could have hastened such genotypic differences between the two sites. Some black pines in Yeocheon have even showed chlorotic mottle and tip necrosis on the needles.
Table 2 shows the photosynthetic pigment content in the needles of damaged and healthy trees in Yeocheon. Chlorophyll content in the current needle did not significantly between damaged and healthy trees, but a significant difference was observed in 1-year-old needle. The reduction in chlorophyll content could be the result of deficiency in nutrients such as nitrogen (Oren et al. 1993, Tausz et al. 1996) and a decrease in chlorophyll biosynthesis or chlorophyll destruction by oxidative stress (Shimazaki et al. 1980, Heath 1989, Aarti et al. 2006). This is associated with visible symptoms such as chlorotic mottle or tip necrosis on the needle. Typically, reduction in chlorophyll content due to nutrient deficiency occurs easily even in young needles or leaves (Chandler and Dale 1995, Mehne-Jakobs 1996). However, in our study, the difference in chlorophyll content between damaged and healthy trees could not be attributed solely to nutrient deficiency, because we found no significant differences in chlorophyll content between the damaged and healthy needles of the current needles and visible injury symptoms were not observed in the current needles in both groups. On the contrary, the difference in chlorophyll content between the two groups was observed in 1-year-old needles, and visible injury was also observed on 1-year-old needles of damaged trees. We proposed that the chlorophyll reduction observed in this study could be the result of long-term exposure of various oxidative stress rather than nutrient deficiency in the soil as induced by high O3 and SO2 concentrations in Yeocheon compared with other areas.
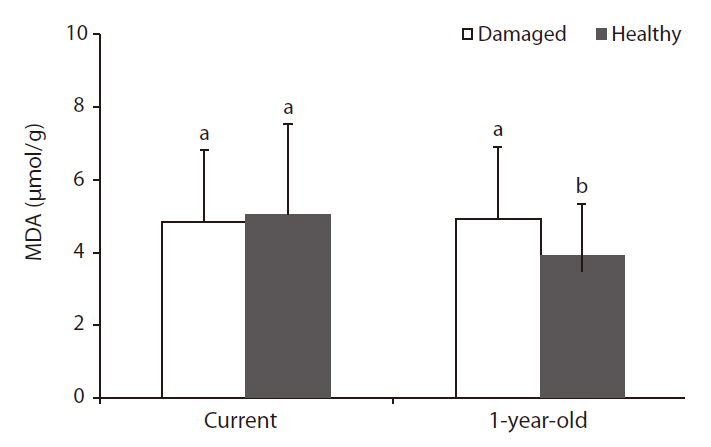
As with the results on chlorophyll content, MDA content, which represents the state of membrane lipid peroxidation (Ranieri et al. 1996), in 1-year-old needles of damaged trees was significantly higher than that of healthy trees, but MDA content in current needles was not significantly different between damaged and healthy trees (Fig. 4). The increase of MDA content in 1-year-old needles of damaged trees was considered to be the effects of long-term exposure to oxidative stress during the growth period, and an increase in lipid peroxidation indicates that damage was occurring at the membrane levels (Calatayud et al. 2006, Han et al. 2009). In a study on age-related peroxidation in annual beech (

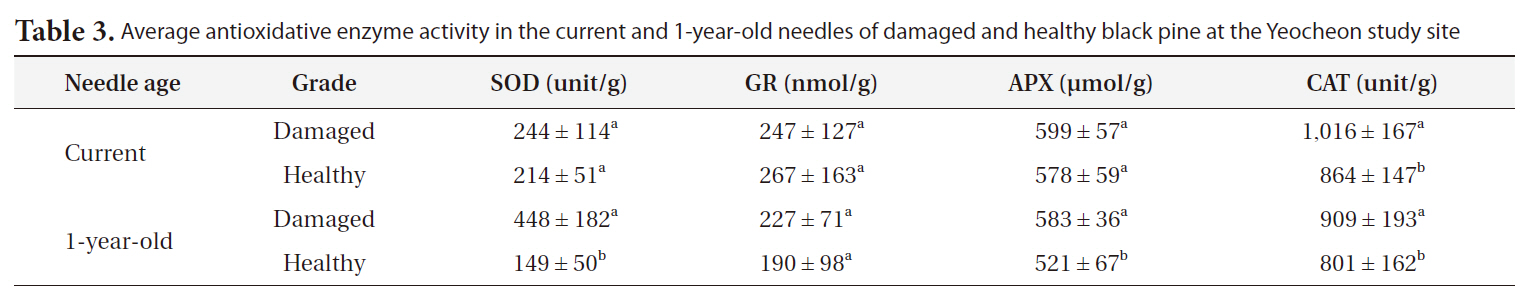
Average antioxidative enzyme activity in the current and 1-year-old needles of damaged and healthy black pine at the Yeocheon study site
Mill.) leaves and perennial fir (Abies alba Mill.) needles, Kunert and Ederer (1985) reported that older needles are strongly associated with a higher peroxidation of lipids.
In addition, significant differences in the activities of antioxidative enzymes were shown between damaged and healthy trees in 1-year-old needles, and not in current needle (Table 3), which correlates with the results of lipid peroxidation. It has been reported that chlorotic spruce needles contain higher ascorbate and glutathione levels than green needles (Osswald et al. 1987). In chlorotic needles, the level of total ascorbate and the activities of superoxide dismutase, monodehydroascorbate radical reductase, NAD-malate dehydrogenase, and glucose-6-phosphate dehydrogenase were significantly increased (Polle et al. 1992). These results may indicate that the extent of age-related peroxidative damage of cells seems to be controlled by the potency of antioxidative systems (Kunert and Ederer 1985).
In conclusion, the growth reduction and damage observed on black pine in Yeocheon could be the result of oxidative stress, and of the additional expense of photosynthate as a consequent defense mechanism against oxidative stress due to high ozone and SO2 concentration in the atmosphere.