Like many other species, reptile species are declining throughout the world, and some of them are endangered(Gibbons et al. 2000). Based on the 2009 annual Red List report compiled by the International Union for Conservation of Nature and Natural Resources (IUCN), more than 470 reptile species are currently classified as endangered(IUCN 2009). In Korea, many reptile species are also in decline, although few results have been published in this regard (Song 2007). In order to successfully conserve or restore a particular species, knowledge of its life history and basic ecology, including physical characteristics of individuals and age structures of natural populations, is a matter of critical importance (Germano 1992, Andreone et al. 2005). Nevertheless, very few studies concerning reptile species have been conducted (Anderson and Vitt 1990, Shine and Charnov 1992); this is particularly true of reptile species in Korea.
The Mongolian racerunner (
Skeletochronology has been used to determine the ages of many different amphibian and reptile species (Castanet and Baez 1988, 1991, Cheong et al. 2007, Lee and Park 2009, Lee et al. 2009) based on the number of lines of arrested growth appearing in cross-sectioned phalanges. Lines of arrested growth are formed as the result of different growth rates of phalange bones in active growing seasons relative to those in resting winter seasons (Patnaik 1994, Esteban et al. 2004). In lizards, it has been previously reported that ages estimated by skeletochronology deviate by one or two years from ages calculated via the mark-recapture method (Smirina and Tsellarius 1996). Nevertheless, skeletochronology has been frequently employed in reptile studies to determine age at sexual maturity, individual growth coefficients, and age structures of natural populations (Castanet and Baez 1988, Halliday and Verrell 1988, Roitberg and Smirina 2006). This method is rather attractive because several years are required to obtain adequate data via the mark-recapture method.
In this study, we evaluated the physical characteristics of neonate, female, and male Mongolia racerunners collected from a population located in Taean-gun, Chungcheongnam-do, South Korea, and determined the age structure of the population.
On July 10, 2008 and June 11, 2009, we collected 67 female and 50 male Mongolian racerunners by hand or with an insect net in a natural population located in Baramare Beach (N 36°24′34.9″, E 126°23′01.4″), Taean-gun, Chungcheongnam-do, South Korea. The Baramare population is located on a small sand dune island (23 m long × 17 m wide) approximately 50 m from the coastline. Magellan wheatgrass (
Whenever we captured a lizard, we first determined the sex of the lizard based on the presence of a hemipenis (male) or bite marks on the abdomen (female). After sex determination, we measured the snout-vent length (SVL), head length, head width, and body mass of each lizard with a digital vernier caliper (M500-181; Mitutoyo, Tokyo, Japan) and a digital field balance (TMB 120-1; Kern, Wildeck, Germany) to the nearest 0.1 mm and 0.1 g, respectively. In order to determine the ages of individuals by skeletochronology, we clipped the outermost two segments of the central toe from the right hindlimb and individually preserved each in 10% neutral-buffered formalin.
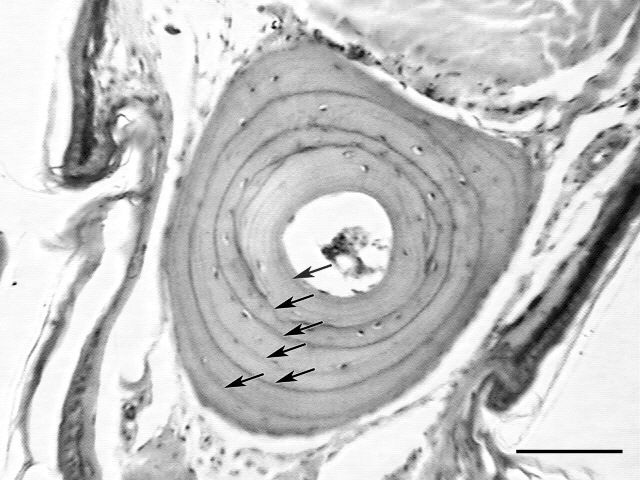
Skeletochronology was conducted according to the methods developed by Cheong et al. (2007) and Lee and Park (2009). First, we washed the clipped toes for 24 h in running tap water to clean them. We then softened the toe bone by submerging it for 4 h in 5% nitric acid. Subsequently, the dehydrated toes were paraffin-embedded and sectioned at a thickness of 15 ㎛ using a rotary microtome (Erma, Tokyo, Japan). The collected sections were then stained with Harris’ hematoxylin & eosin and observed under a microscope. Growth zones and lines of arrested growth (LAGs) were clearly visible in the cross-sections of the phalanges (Fig. 1). The number of LAGs was determined independently by two authors based on the methods developed by Cheong et al. (2007) and Lee and Park (2009). The two age estimates were compared to derive a consensus value for the age of each individual.
To obtain SVL data for neonates, which was necessary to estimate the growth curves of the female and male Mongolian racerunners, we collected 10 pregnant females from the Baramare field population on May 12, 2009 and brought them into the laboratory. We housed them in two plastic boxes (48 cm long × 27 cm wide × 30 cm deep) containing sand with a depth of 5-7 cm, wet paper towels that provided hiding places for the lizards, and a ceramic water container (5 cm diameter, 5 cm deep). Approximately 10 days after housing, the females began to oviposit eggs inside the sand. We successfully collected a total of 38 eggs. We placed the eggs in an incubator (35 cm long × 20 cm wide × 7 cm deep, PX-20R; Auto Elex Co., Ltd, Seoul, Korea) containing wet vermiculite at a depth of approximately 5 cm. The air temperature and relative humidity within the incubator were maintained at 27.5-29oC and 80-90%, respectively, throughout the incubation period. After approximately 50 days of incubation, most of the eggs had successfully hatched. We measured the SVL, head length, head width, and body mass of 25 neonates using a digital vernier caliper and a digital balance five days after hatching.
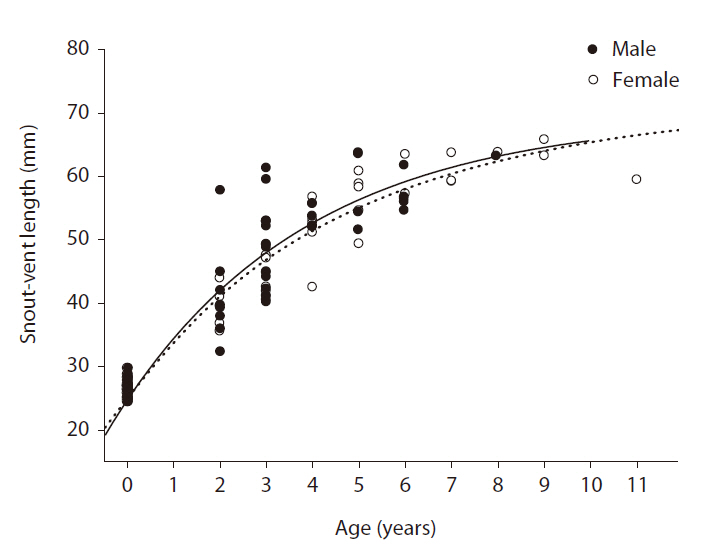
Growth curves of the female and male Mongolian racerunners were estimated by applying the growth curve model of von Bertalanffy (1938) to the SVL data for females and males recorded in the field and for neonates hatched in the laboratory. The von Bertalanffy model equation is St = Sm - (Sm - So) e?k (t - to), where St = average SVL of females or males at age t, Sm = asymptotic SVL of females or males, S0 = average SVL of neonates, t = age of each individual, t0 = age at which offspring hatch, and K = growth coefficient of female or male SVL (average growth rate of SVL per year). For the So value, we used 26.6 mm (calculated from 25 neonates); for to, we used 0.15 because the egg incubation period was approximately 50 days in this species. The von Bertalanffy growth model was fitted to the average growth curves using dynamic fitting in SigmaPlot ver. 10.0 (Systat Software Inc., Houn-
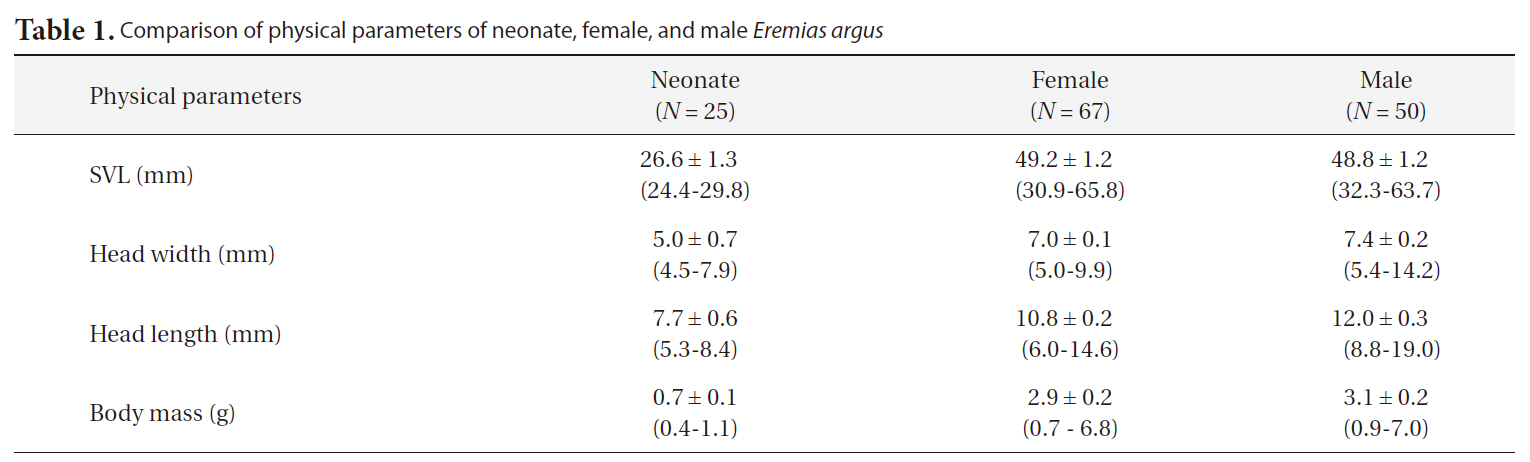
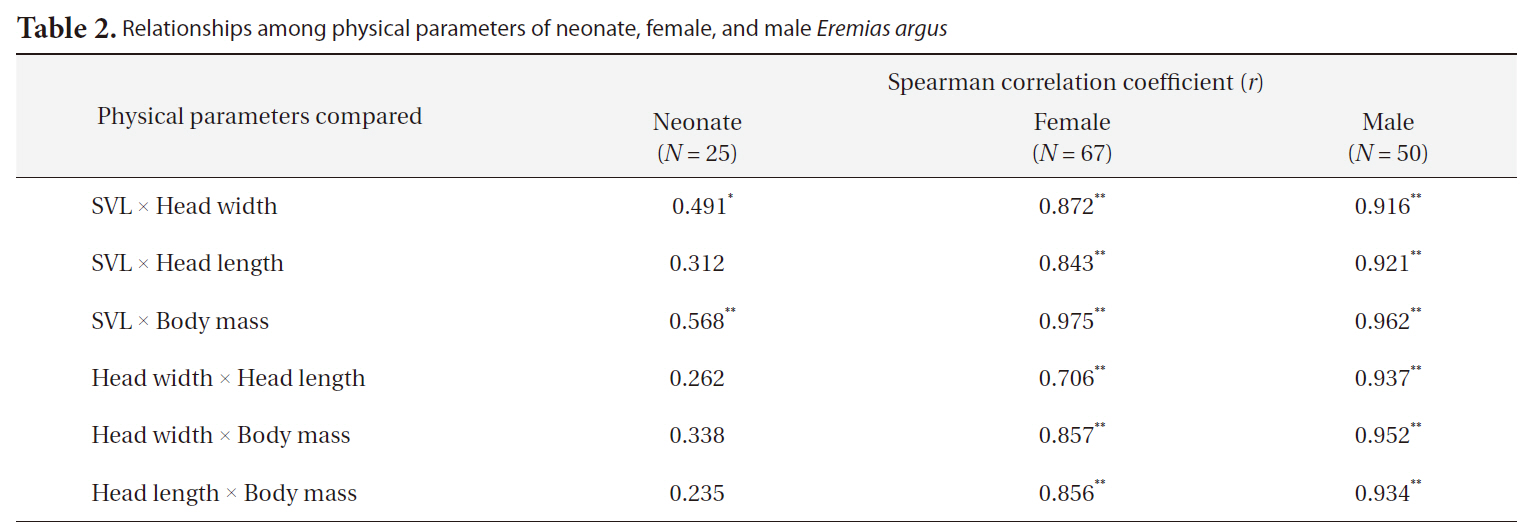
[Table 1.] Comparison of physical parameters of neonate female and male Eremias argus
Comparison of physical parameters of neonate female and male Eremias argus
slow, UK).
Among the physical parameter data, only the SVL data passed the normality test after square root data transformation (Kolmogorov-Smirnov,
The SVLs of neonates, females, and males were approximately 26.6 mm, 49.2 mm, and 48.8 mm, respectively. Interestingly, the head lengths of the males were significantly greater than those of females (ANCOVA, F = 20.28, df = 1,
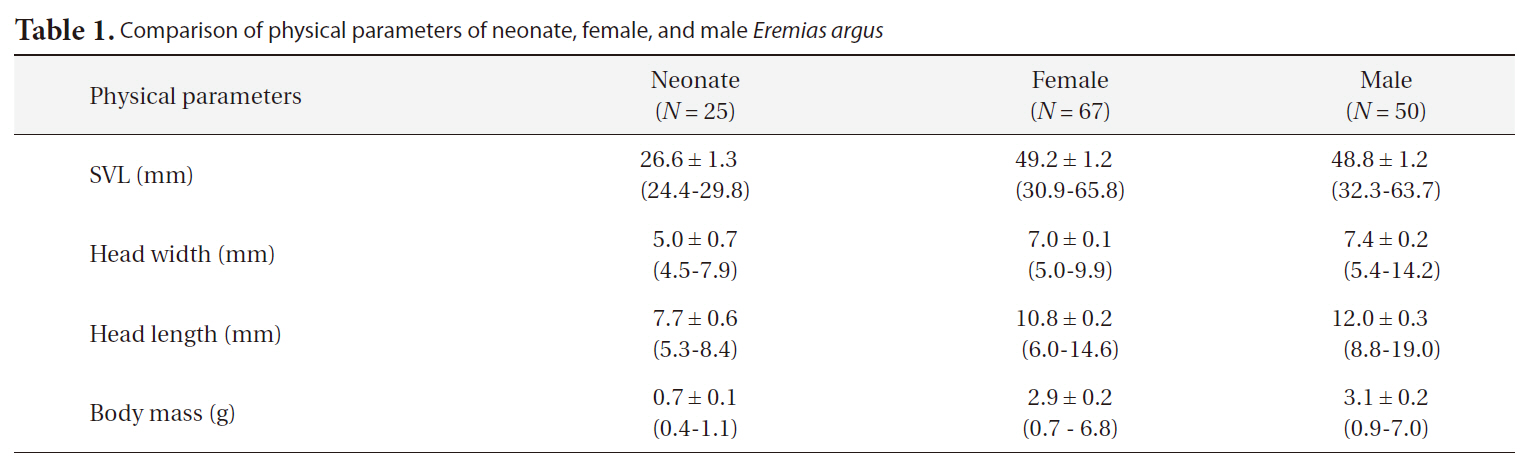
[Table 2.] Relationships among physical parameters of neonate female and male Eremias argus

Relationships among physical parameters of neonate female and male Eremias argus
nificant in all comparisons made (Spearman correlation test,
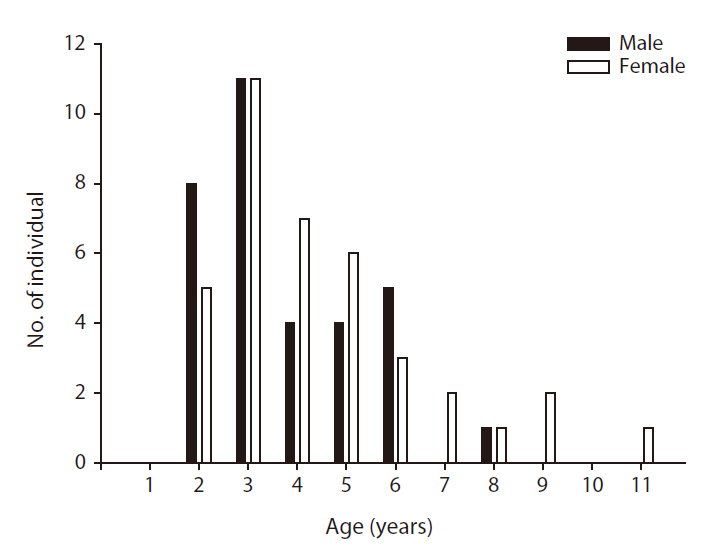
We successfully determined the ages of 38 females and 33 males among a total of 117 lizards, but were unable to determine the ages of 46 lizards due to a loss of sections during the skeletochronology process or incomplete staining of sections. The ages of the females ranged from two to eleven years (mean ± SE = 4.5 ± 0.4,
In this study, we evaluated the physical characteristics of female and male Mongolian racerunners and determined the age structure of a natural population. The heads of the male lizards were significantly longer than those of the females, but no other physical parameters differed between the females and males. The ages of the natural population of Mongolian racerunners tested herein ranged between two to eleven years, and the numbers of females and males did not differ within the different age classes. Although asymptotic SVL did not differ between females and males, the males evidenced a higher growth coefficient than the females.
In lizards, sexual dimorphism is generally promoted by sexual selection and scarcely by natural selection such as food competition (Shine 1989, Vincent and Herrel 2007). Because the larger heads (usually both longer and wider) of male lizards may be a mechanism of relative advantage in male-male mating competitions and in subduing potential mates during mating (Olsson et al. 2002), the males of several species, including the Multi-ocellated racerunner (
In contrast to other lizard species in which females evidence longer SVL than males (Haenel and John-Alder 2002, Liu et al. 2008), our finding that SVL did not differ between female and male Mongolian racerunners implies that female body size may not constitute a reliable indicator of female fecundity. Similar results have been recently reported in studies of E. multiocellata (Li et al. 2006). In that species, females’ SVLs were not correlated with litter size, and explained only 19% of the variability in litter mass, thereby suggesting that selective pressure on large female SVL for high fecundity through sexual selection is quite low. Such low selective pressure on female SVL should not be sufficient to induce differences in SVL between females and males (Li et al. 2006). By way of contrast, in lizards such as the eastern fence lizard (
Based on our skeletochronological findings, the lifespan of Mongolian racerunners is approximately 10 years. This lifespan is greater than that of
The higher SVL growth coefficient of male Mongolian racerunners as determined in our study may explain, at least in part, the finding of greater head length in males relative to females. The results of previous studies have shown that male