Yukmigeehwang-hwan extracts (Liuweidihuang-wan in chinese, YHE) are composed of six herbal medicines1). YHE is classic decoction for the treatment of Kidney and Liver yin deficiencies such as a general weakness of the lower back and legs, a light-headedness, vertigo, tinnitus and diminished hearing1,2).
Neutrophil elastase (or leukocyte elastase) is a serine protease in the same family as chymotrypsin and has broad substrate specificity. As with other serine proteases it contains a charge relay system composed of the catalytic triad of histidine, aspartate, and serine residues that are dispersed throughout the primary sequence of the polypeptide but that are brought together in the three dimension conformation of the folded protein. The gene encoding neutrophil elastase, ELA2, consists of five exons. It breaks down elastin, an elastic fiber that, together with collagen, determines the mechanical properties of connective tissue3). This neutrophil elastase (NE) is a potent non-specific serine protease which plays a role as bactericidal agent in the degradation of immune complexes by intraphagosomal processes. It promotes inflammation, pulmonary emphysema, and chronic obstructive pulmonary disease4-7). The proposed pathogenesis of emphysema development involves a combination of inflammation, elastase, matrix metalloprotease imbalance, apoptosis, and oxidative stress8). It is generally accepted that pulmonary exposure to porcine pancreatic elastase (PPE), an enzyme that acts predominantly on elastin9), elicits acute lung inflammatory response with neutrophils and macrophages10-12). Clinical studies for human pulmonary hypertension (PH) and systolic left ventricular failure are now in progress as well4-7).
The production of reactive oxygen species (ROS) was induced by NE13,14). They reported that NE enhancement of MUC5AC messenger RNA levels was dependent on the production of intracellular oxidants or an alteration in the redox state of the cell. It means that ROS may play a role in elastase mediated inflammation. Nitric oxide plays an pivotal role in elastase mediated diseases as well15).
In the present study, I investigated the effects of YHE on elastase activity. Anti-oxdative activities of YHE were also examined via measuring the DPPH free radical scavenging and nitrite scavenging activities .
The Rehmanniae Radix Preparata, Corni Fructus, Dioscoreae Rhizoma, Alismatis Rhizoma, Hoelen, and Moutan Cortex Radicis were purchased from Omniherb (Korea). YHE was prepared as follow. The Rehmanniae Radix Preparata (24 g), Corni Fructus (12 g), Dioscoreae Rhizoma (12 g), Alismatis Rhizoma (9 g), Hoelen (9 g), and Moutan Cortex Radicis (9 g) in 2,000 ml distilled water was heated in a heating extractor for 3 hours. The extract was filtered and concentrated by using the rotary evaporator. The extracts were lyophilized by using freeze dryer (13.7 g). The lyophilized extract was dissolved in water and filtered three times through microfilter paper (Whattman no. 2, 0.45-0.2 μm). It was placed in a disinfected vial and sealed for further study.
All reagents were purchased from Sigma-Aldrich(St. Louis, MO, USA).
3. Elastase activity inhibition
The elastase activity was evaluated by using a modification of a previously reported method of Kraunsoe et al16). In order to evaluate the inhibition of elastase activity, the amount of released p-nitroan iline, which was hydrolyzed from the substrate, Nsuccinyl-Ala-Ala-Ala-p-nitroanilide, by elastase,was read with a maximum absorbance at 410 nm17).In brief, 2 mM N-succinyl-Ala-Ala-Ala-p-nitroanilide was prepared in a 0.1 M Tris-Cl buffer (pH 8.0),and this solution was added to the stock sample.Each sample solution was diluted to final concentrations of 0.01, 0.1, and 1 mg/ml. The solutions were mixed thoroughly by tapping before an elastase(0.1360 unit/ml) stock solution was added.Solution was incubated for 10 min at 37°C, and the absorbance was measured at 410 nm. The percent scavenging capability was calculated according to the following equation:
Elastase inhibitory activity (%) =
[(OD410 of control) - (OD410 of sample) / (OD410 of control)] × 100
4. DPPH free radical scavenging activity
The scavenging effect of sample on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals was assayed according to the procedure described by Shimada et al18). The DPPH radical shows a deep violet color due to its unpaired electron, and radical scavenging capacity can be followed spectrophotometrically by the loss of absorbance at 540 nm17). In brief, sample was added to 1 ml of freshly prepared ethanolic solution containing a final DPPH radical concentration of 0.2 mM. After it stood for 30 min in the dark, the absorbance of the mixture was measured at 540 nm against an ethanol control with a spectrophotometer.The percent scavenging capability was calculated according to the following equation:
DPPH free radical scavenging activity (%)
= [(OD540 of control) - (OD540 of sample) / (OD540 of control)] × 100
5. Nitrite scavenging activity
Nitrite scavenging activity (NSA) of sample was determined by using Griess reagent19). First, 1 ml of sample was mixed with 1 ml of 1 mM sodium nitrite. The mixture was added to 8 ml of 0.2 M citrate buffer (pH 1.2, 3.0, and 6.0) and incubated for 1 h at 37°C. Then, 1 ml aliquot was removed and added to 2 ml of 2% acetic acid and 0.4 ml of Griess reagent (1% sulfanilic acid and 1% naphthylamine in a methanol solution containing 30%acetic acid). After vigorous vortex mixing, the mixture was placed at room temperature for 15 min and the absorbance was measured at 520 nm. The NSA(%) was calculated by the following equation.
NSA (%)=[1 - (A - C) / B] × 100
Where, A is the absorbance of treated sample, C is the absorbance of sample, and B is the absorbance of 1 mM NaNO2.
The results were expressed as means ± standard error of the mean (SEM). Significances of changes were evaluated using the Students' t-test. Values of p ? 0.05 were considered significant.
1. Inhibition of the elastase activity
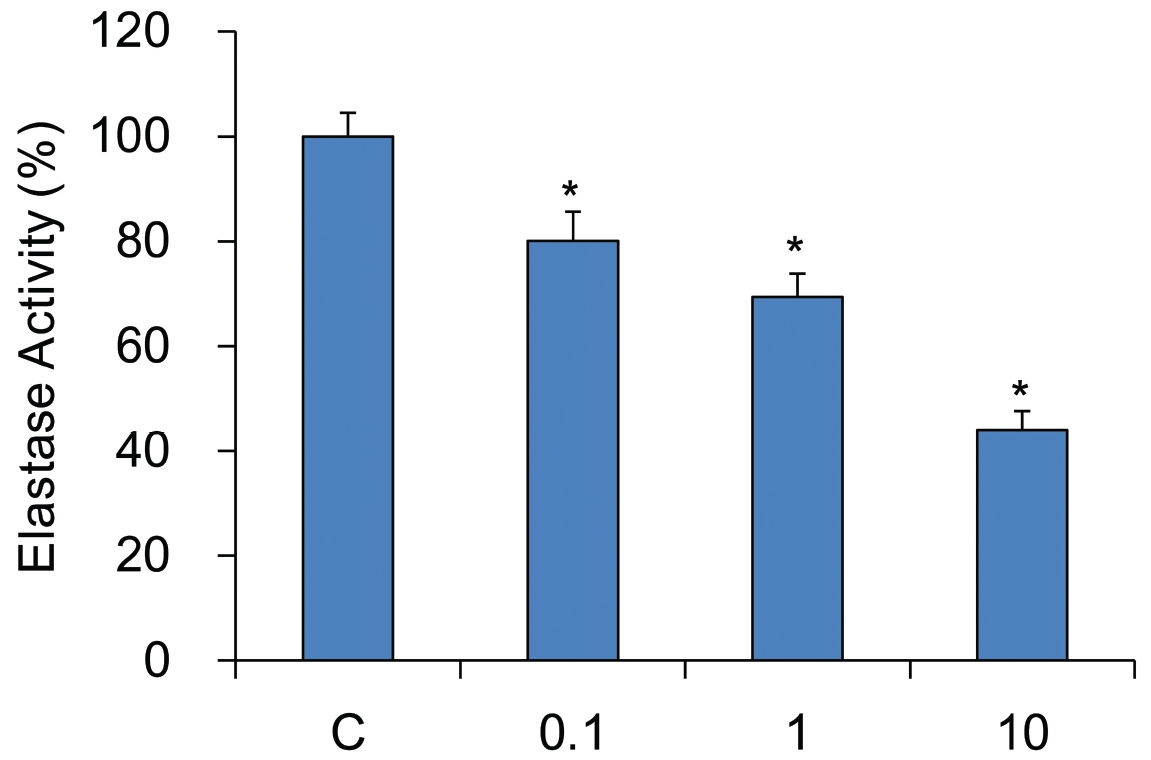
The inhibitory effect of YHE on elastase activity was determined according to the method described previously. YHE showed the elastase inhibitory effect in dose dependent manner. YHE was found to inhibit elastase activity significantly at all concentrations of YHE 10 mg/ml, 1 mg/ml and YHE 0.1 mg/ml treated groups (44.0 ± 3.7%, 69.4 ± 4.5% and 80.0 ± 5.5% compared with 100% elastase activity of control group respectively, Fig.1 ).
2. DPPH free radical scavenging capability
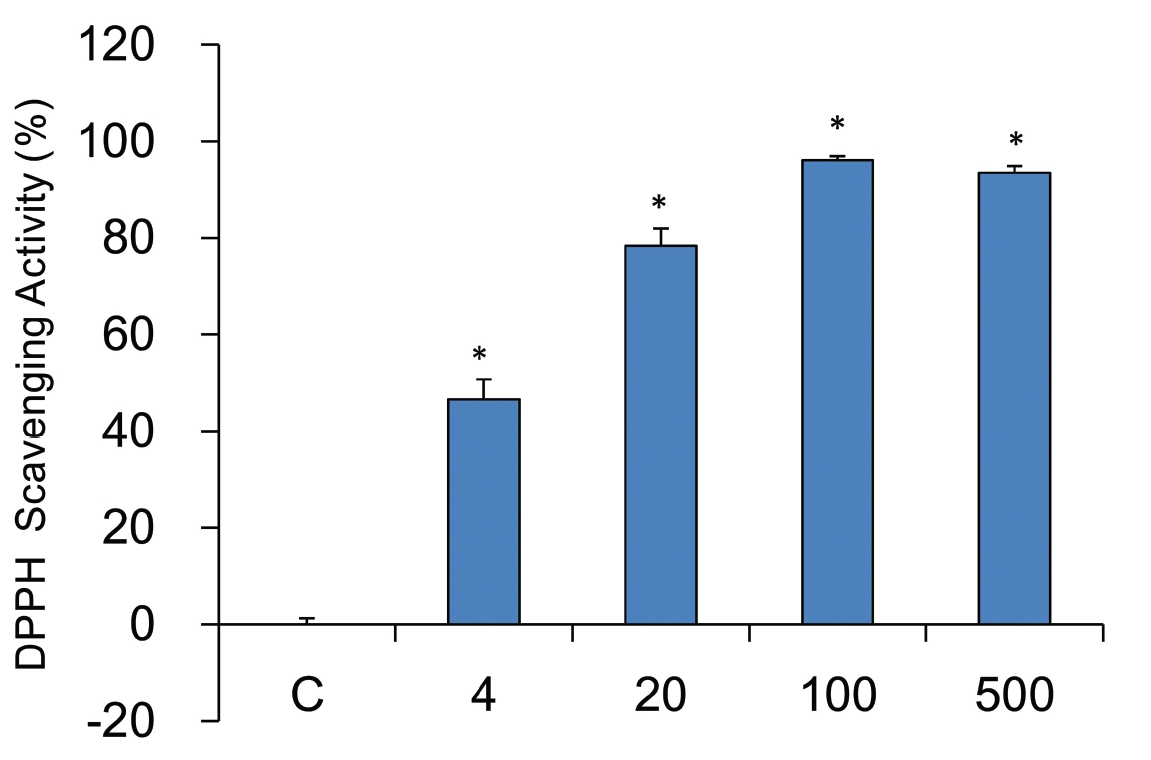
It has been reported that reactive free radicals was induced by elastase in neutrophil and that they play a role in inflammation. Assays of the free radical scavenging capacity were carried out by the DPPH method. The free radical scavenging capacity of sample was measured at each concentration (0, 4, 20, 100, and 500 mg/ml). A dose dependent free radical scavenging capability was observed in sample treated groups. YHE 500 mg/ml treated groups had the highest scavenging capability, of 93.5 ± 1.4%, while 100, 20, and 4 mg/ml treated groups had 96.1 ± 0.9%, 78.4 ± 3.6%, and 46.6 ± 4.1%, respectively (Fig.2 ).
3. Nitrite scavenging capability at pH 1.2
Nitrite scavenging capability changes at various pH environments. Accordingly, nitrate scavenging activities at pH 1.2, 3.0, and 6.0 were measured in this study.
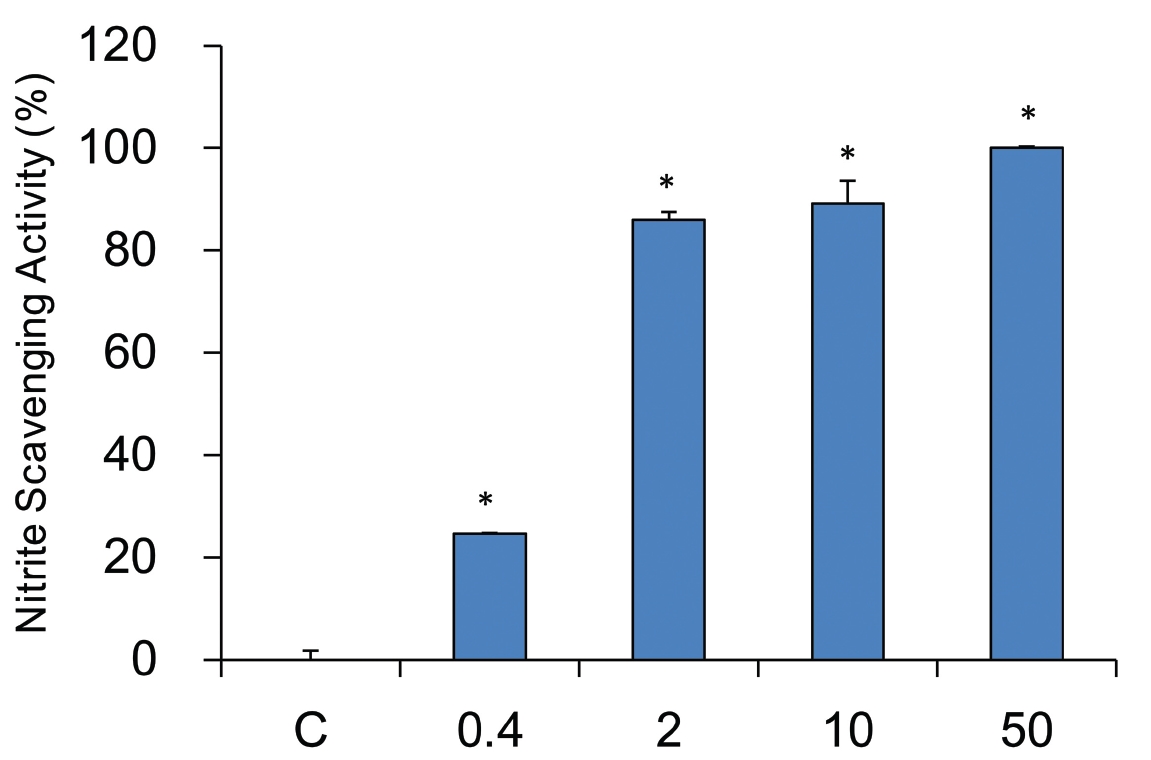
The nitrate scavenging capacity of sample was measured at each concentration (0, 0.4, 2, 10, and 50 mg/ml). A dose dependent nitrate scavenging capability was observed in sample treated groups. All tested concentraions showed the statistical significances. YHE 50 mg/ml treated groups had the highest scavenging capability, of 100.0 ± 0.3%, while 10, 2, and 0.4 mg/ml treated groups had 89.1 ± 4.4%, 86.0 ± 1.5%, and 24.7 ± 0.1%, respectively (Fig.3 ).
4. Nitrite scavenging capability at pH 3.0
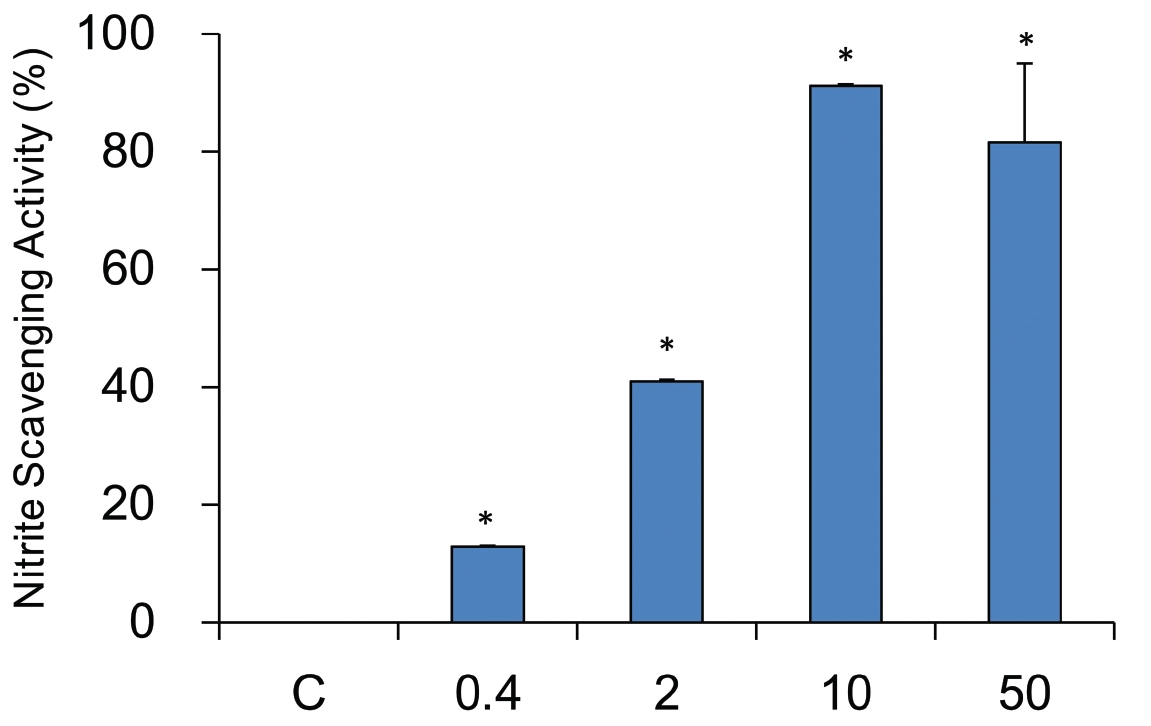
The nitrate scavenging capacity of sample was measured at each concentration (0, 0.4, 2, 10, and 50 mg/ml). A dose dependent nitrate scavenging capability was observed in sample treated groups. All tested concentraions showed the statistical significances. YHE 10 mg/ml treated groups had the highest scavenging capability, of 91.3 ± 0.3%, while 50, 2, and 0.4 mg/ml treated groups had 81.6 ± 13.5%, 41.0 ± 0.3%, and 12.9 ± 0.1%, respectively (Fig.4 ).
5. Nitrite scavenging capability at pH 6.0
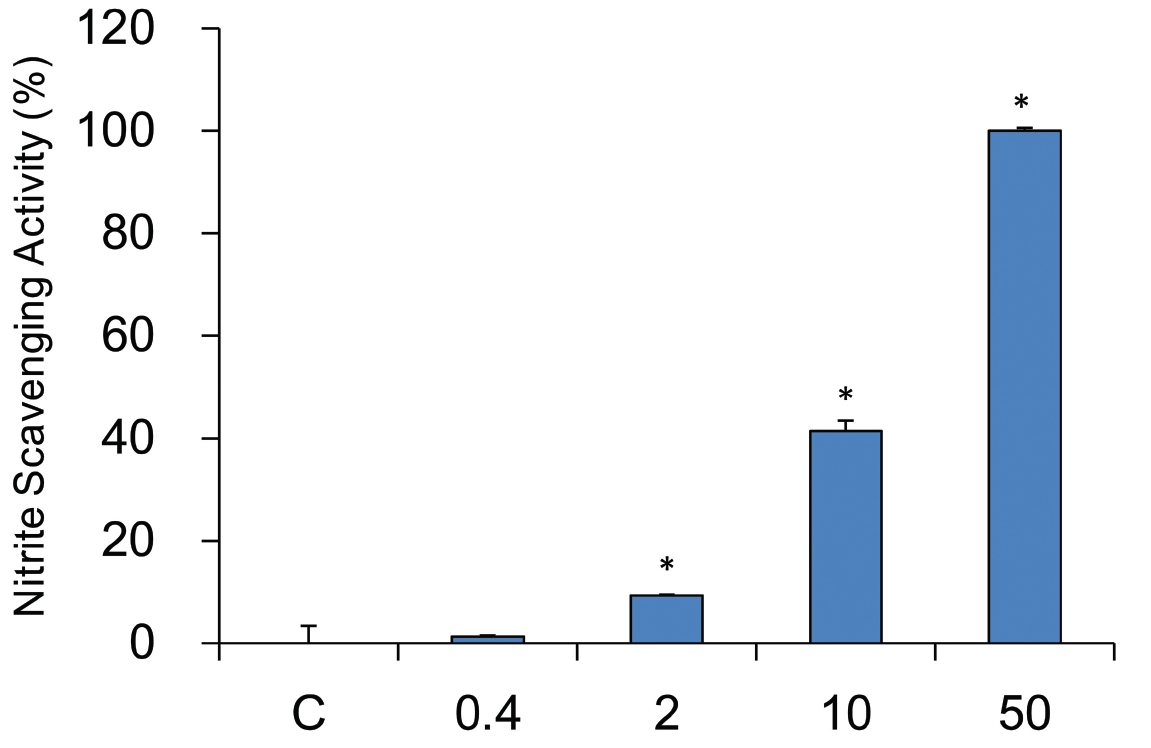
The nitrate scavenging capacity of sample was measured at each concentration (0, 0.4, 2, 10, and 50 mg/ml). A dose dependent nitrate scavenging capability was observed at pH 6.0 environment as well. Three concentrations of YHE treatment (2, 10, and 50 mg/ml) showed the statistical significances. The scavenging effects of 0, 0.4, 2, 10, and 50 mg/ml are 1.3 ± 0.3%, 9.3 ± 0.3%, 41.5 ± 2.0%, and 100.0 ± 0.5%, respectively (Fig.5 ). Considering data from Figure 3 to 5, nitrite scavenging capability was varied with increasing pH, suggesting it is pH dependent. Accordingly, pH 1.2 could be the best environment for nitrite scavenging activity of YHE. However, all tested pH showed the effective nitrite scavenging capability.
YHE is composed of six herbal medicines. The Rehmanniae Radix Preparata, Corni Fructus, Dioscoreae Rhizoma, Alismatis Rhizoma, Hoelen, and Moutan Cortex Radicis are ground into powder and formed into small pills with honey1). YHE is classic decoction for the treatment of Kidney and Liver yin deficiencies. When Kidneys are weakened, the marrow will become depleted and there will be a general weakness of the skeletal structure which is focused in the area of the body, most closely associated with the Kidneys, the lower back and legs. The eyes are nourished by the Liver; lack of nourishment manifests itself as light-headedness and vertigo. The ears are nourished by the Kidneys; lack of nourishment manifests itself as tinnitus and diminished hearing1,2).
Elastase breaks down elastin, an elastic fiber that, together with collagen, determines the mechanical properties of connective tissue. The neutrophil form breaks down the outer membrane protein A (OmpA) of E. coli and other Gram-negative bacteria, and also breaks down Shigella virulence factors. This is accomplished through the cleavage of peptide bonds in the target proteins. The specific peptide bonds cleaved are those on the carboxy side of small, hydrophobic amino acids such as glycine, alanine, and valine. Actually, elastase is the only enzyme that is capable of degrading elastin, an insoluble elastic fibrous protein in animal connective tissues. It is capable of hydrolyzing nearly all proteins, including supporting and structural proteins of the connective tissue such as collagen and elastin20). Elastin is the main component of the elastic fibers of the connective tissue and tendons. The elastic fibers in the skin, together with the collagenous fibers, form a network under the epidermis21). Elastase also plays a critical role in inflammatory processes. The enzyme has drawn much attention, primarily because of its reactivity and non-specificity. It is able to attack all major connective tissue matrix proteins, including elastin, collagen, proteoglycans, and keratins22). However, recent observations indicate that the role of neutrophil elastase (NE) in inflammation is more complex than the simple degradation of extra-cellular matrix components. This NE is a potent non specific serine protease which plays a role as bactericidal agent and in the degradation of immune complexes by intraphagosomal processes. It promotes inflammation when the granule contents are secreted in the extracellular environment. In certain pathological circumstances, an imbalance between NE and its major plasmatic inhibitor alpha 1-PI (formerly, alpha 1-antitrypsin) leads to abnormal tissue destruction and disease development. Genetic or acquired alpha 1-PI deficiency is thought to be involved in the pathogenesis of pulmonary emphysema. A variety of degenerative and degradative disorders are also associated to uncontrolled proteolysis by NE (rheumatoid arthritis, glomerulonephritis, adult respiratory distress symptom, psoriasis, cancer). Recent studies suggest that NE not only plays a key role in lung destruction (emphysema) but can also modulate proliferative changes (fibrosis) in inflammatory processes. Thus, NE could be considered to have potential multiple roles in the pathogenesis of both emphysema and lung fibrosis23). Numerous inhibitors of NE have been reported. Various molecules are currently undergoing clinical trials for emphysema and other pulmonary diseases24). The defects of elastic matrix aggravate hypertension which is associated with alteration in the great arteries, arteries, and arterioles. Clinical studies for human pulmonary hypertension (PH) and systolic left ventricular failure are now in progress. An elastase inhibitor is currently being investigated in phase I clinical trials in patients with PH owing to chronic obstructive pulmonary disease4-7).
In this study, inhibitory effect of YHE on elastase activity was determined according to the method. YHE showed the elastase inhibitory effect in dose dependent manner. YHE was found to inhibit elastase activity highly at a concentration of 10 mg/ml.
The production of Reactive oxygen species (ROS) was induced by NE13,14). They reported that NE enhancement of MUC5AC messenger RNA levels was dependent on the production of intracellular oxidants or an alteration in the redox state of the cell. It means that ROS may play a role in elastase mediated inflammation. Accordingly, anti-oxdative activities of YHE were also examined. DPPH free radical scavenging capability of YHE was measured at each concentration (0, 4, 20, 100, and 500 mg/ml). A dose dependent free radical scavenging capability was observed.
Nitric oxide plays an pivotal role in elastase mediated diseases15,25). So, nitrite scavenging activities were also examined. However, nitrite scavenging capability changes at various pH environments. Accordingly, nitrate scavenging activities at pH 1.2, 3.0, and 6.0 were measured in this study. Considering data at pH 1.2, 3.0, and 6.0, nitrite scavenging capability was varied with increasing pH, suggesting it is pH dependent. The pH 1.2 could be the best environment for nitrite scavenging activity of YHE. However, all tested pH showed the effective nitrite scavenging capability.
In conclusion, YHE showed the inhibiting effects on the elastase, and free radical scavenging capability of DPPH and nitrite. These results suggest that YHE may have potential effects for pulmonary emphysema and pulmonary hypertension. Further studies might be needed to unravel exactly under the clinical trial and mechanisms.