


Fullerene and its derivatives have been interested by the field of medicinal chemistry and material science because they have the properties of DNA-cleavage, enzyme-inhibiting, and radical quenching by producing singlet oxygen with the visible range of light as well as electron acceptor [1-4]. The cycloaddtion of fullerenes to be known one of method among the useful methods to get the fullerene derivatives has attracted by chemist, due to unique chemical reactivity and characteristic for produce at least more than two structural isomers [5]. In particular, the 1,3-dipolar cycloaddition of organic azides to fullerenes, it is well known that this reaction involved a [3+2] cycloaddition of azide to the fullerenes with formation of intermediate triazoline, followed by thermal cleavage of N2 affording the opened [5,6]-bridged azafulleroid and [6,6]-bridged aziridinofullerene depending on the nature of the substituent of azides [6,7].
Ultrasonic processing was applied to the science, engineering technology, medicinal field and also used to the synthesis of fullerene derivatives [8,9] as an energy source, since it has known to cause chemical reactions. The sonochemical reactions are promoted by cavitation of liquid caused by ultrasonic waves in the traveling in the solution. Here, cavitation implies the formation of micro-bubbles in a liquid subjected to sonication, which implode and generate high pressures and temperatures in their surroundings [10,11].
The self-assembly of metal nanoparticles in multilayer films is one of the interesting research fields in materials science due to the development of nanotechnology [12]. The self-assembly of nanomaterial is mostly based on electrostatic interactions between adsorbed monolayers of nanomaterial and oppositely charged linker molecules. The electrochemical studies on these multilayers revealed the compact nature of the surface assemblies. Here, we report synthesis of aziridino[70]fullerene derivative with [70]fullerene and methylazidoacetate under ultrasonic irradiation and preparation of self-assembled of [70]fullerene derivative-gold nanoparticle films.
2.1. Chemicals and instruments
[70]Fullerene used in this work was Golden grade from Hoechest and Southern Chemical Group Inc. All solvents and chemical reagents were purchased from Aldrich and Fluka. The ultrasonication of all samples was conducted in continuous mode with an ultrasonic Generator UG1200 made by Hanil Ultrasonic Co. Ltd. Ultrasonic equipment employed in this research having frequency of 20 kHz, power 750W. The configuration of the equipment is horn system, and the
Cycloaddition of methyl azidoacetate (1) with [70]fullerene.
size of the horn tip is 13 mm in diameter. 1H- and 13C-NMR spectra were recorded with a Varian Inova AS400 and Bruker avance digital 400 spectrometers on solutions in CDCl3 with tetramethylsilane as the internal standard. FAB mass spectrum were measured on a JMS-700 Mass spectrometer (JEOL, Japan) instrument using metanitrobenzyl alcohol (NBA) and polyethylene glycol as a matrix, respectively. The UV?vis spectra were obtained by a Varian Cary 100 UV?visible spectrophotometer. Flash chromatography was carried out with silica gel (E. Merck, Art 9385, 230~400 mesh). The reactions were monitored by thin layer chromatography (TLC). TLC was performed using precoated silical gel plates (60F-254, E. Merck) and the spots were detected by charring with 5% sulfuric acid in ethanol.
2.2. Cycloaddition of methyl azidoacetate (1) with [70]fullerene under ultrasonic irradiation
Methyl azidoacetate (1, 27 mg, 0.18 mmol) were added to a solution of C70 (100 mg, 0.12 mmol) in benzene (50 mL). The mixture was irradiated for 2 days under ultrasound irradiation (ultrasonic frequency 20 kHz) and reactions keep temperature at 25~43℃. The solvent was evaporated in vacuum and the crude mixture was separated by flash chromatography (SiO2 column, eluted with toluene/hexane 10/1, v/v) to give the product 2 in 9.1% yield. Unreacted C70 was recovered at the first stage of the chromatography (eluent: toluene).: Rf 0.29 (Tol:Hex 5/1); 1H-NMR (400 MHz, CS2-20% C6D6) δ 5.23 (s, 2 H), 4.67(s) 3.83(s, 3 H) 3.71(s) 3.54(s).; UV-vis λmax nm (cyclohexane): 225, 271, 308, 329, 399, 458; MS (FAB-) [m/z] 928.1 (M+) 840.1(C70).; 13C-NMR (100 MHz, CS2-20% C6D6) of the major adduct of 2 δ 168.07 (C=O), 153.96 151.38 151.00 150.92 150.70 150.59 149.71 149.49 149.37 149.33 149.00 148.77 148.52 148.19 147.37 147.30 147.07 146.33 146.12 144.12 143.86 143.80 143.33 141.56 140.27 139.19 137.96 133.76 133.55 132.10 131.37, 48.78.
4-aminothiophenoxide/hexanethiolate-protected gold nanoparticles with an average core dimension of ~3.2 nm were synthesized using the modified Brust reaction followed by ligand place-exchange reaction of hexanethiolate-protected gold nanoparticles with 4-aminothiophenol [13]. The closed [5,6]-bridged aziridino[70]fullerene derivative-gold nanoparticle films and functionalized gold nanoparticle were selfassembled on the reactive surface such as glass slides functionalized with 3-mercaptopropyl trimethoxysilane. The functionalized glass slides were alternately soaked into the toluene solutions containing the closed [5,6]-bridged aziridino[70]fullerene derivative (10 mM) and 4-aminophenoxide/hexanethiolate-protected gold nanoparticles (~14 μM).
For the application of ultrasound in the synthesis of the closed [5,6]-bridged aziridino[70]fullerene derivative 2, a cycloaddition of methyl azidoacetate (1) with [70]fullerene was performed as described in Scheme 1. First, methyl azidoacetate (1) [14] was reacted with one equivalent of [70] fullerene in benzene under ultrasonic irradiation for 2 days. The silica TLC showed a new reddish-brown spot (Rf = 0.29, Tol:Hex 5/1). After evaporation of the solvent the residue was separated by silica gel flash column chromatography (eluent:toluene/hexane = 10/1, v/v) to give a mixture of monoadducts 2 in 9.1% yield. The [70]fullerene cage can undergo minor decomposition under ultrasonic irradiation, therefore, after the reaction was complete, the majority of the unreacted [70]fullerene could be recovered to be reused in the reaction.
For the characterization of the product 2, FAB-MS, 1Hand 13C-NMR, and UV-vis spectra characterization were performed. The FAB-MS spectrum of 2 from the ultrasonication showed a molecular-ion peak at m/z 928.1(M+) with the base peak for [70]fullerene at m/z 840.1. From the FAB-MS spectrum, 2 was determined to be a 1:1 methyl acetate-[70]fullerene adduct. However, 1H-NMR spectrum showed three signals at 5.23, 3.83, and 4.67, 3.71, and 3.54 ppm for the methyl protons and methylene protons attached to the nitrogen in the ratio of ca. 18.0:4.3:1.0. These were indicated that the product 2 was a mixture composed of
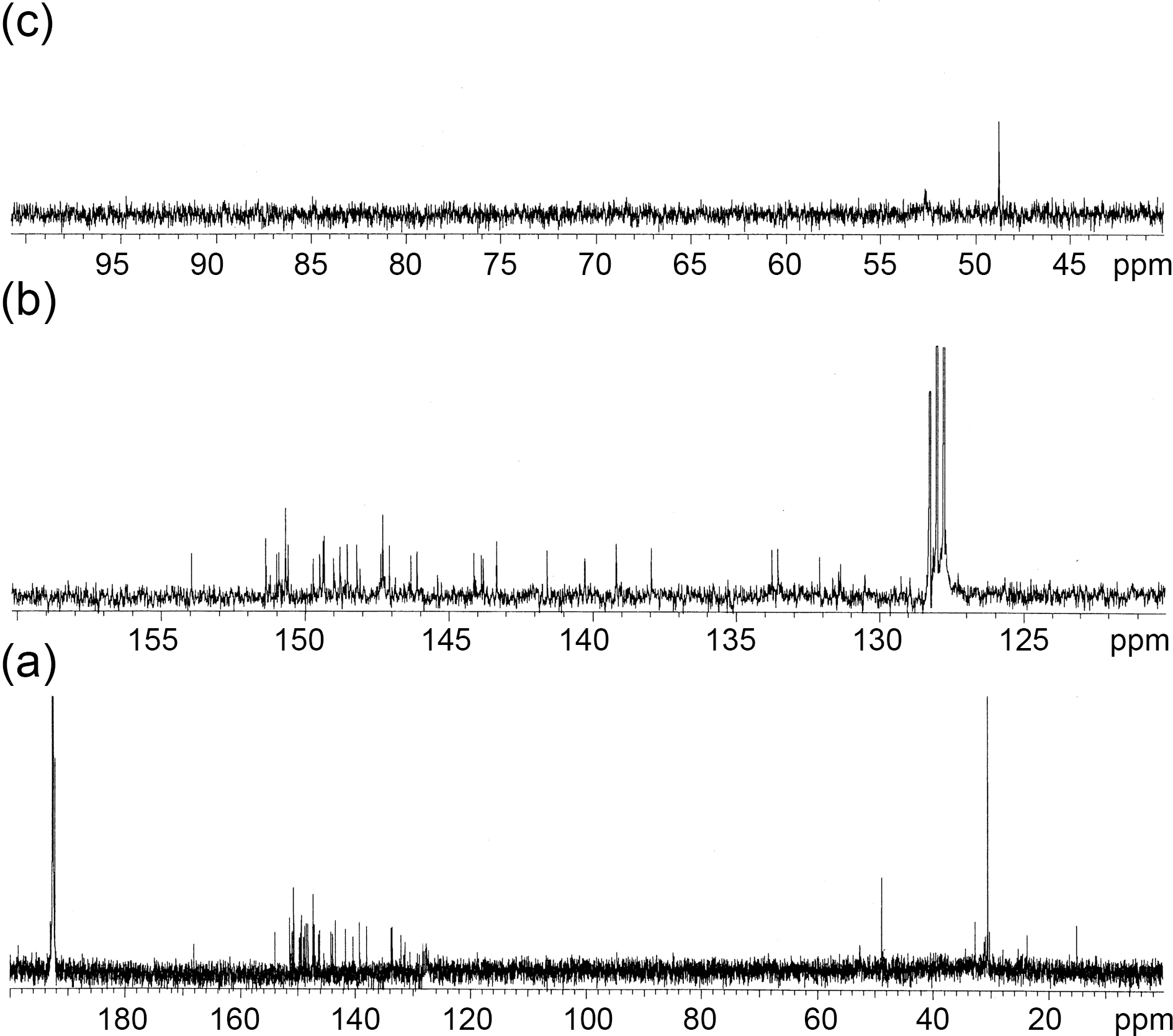
three different types of monoadducts with identical masses.This is in agreement with the fact that cycloaddition to C70 with alkyl azide gave rise to a minimum of four possible isomers[15,16]. To identify the structure of the major adduct in 2, analysis of the UV-vis and 13C-NMR spectra was performed. In the UV-vis spectrum of 2, absorption bands were observed at 225, 271, 308, 329, 399, and 458 nm. This spectrum showed a slight hypsochromic shift relative to C70(UV-vis (cyclohexane): λmax[nm] = 214, 237, 311, 332, 379, 467) that is a highly characteristic absorption pattern for the fulleroaziridine monoadduct (Fig. 1) [15]. The 13C-NMR spectrum of 2 showed at least 31 signals for the fullerene sp2-region indicating the closed [6,6]-bridged isomers, but it did not exhibit two signals for the distinctive sp3-C of fullerene cage (Fig. 2) [15,17]. Moreover, the N-methylene carbon of the major adduct in 2 appeared at 48.78 ppm, while the corresponding carbons for the closed [6,6]-bridged isomer from the thermolysis revealed at 51.54 ppm [16].
In a previous study for the characterization of the glycosyl
[Table 1.] Characterization of Reaction Product between Methyl Azidoacetate (1) and [70]Fullerene
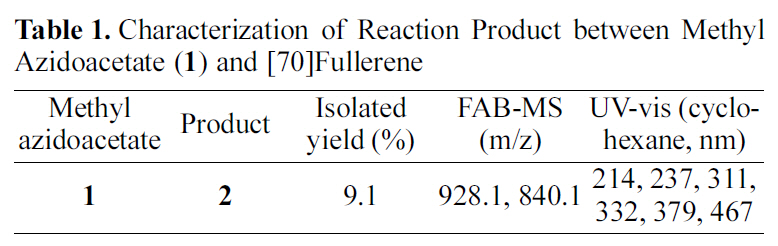
Characterization of Reaction Product between Methyl Azidoacetate (1) and [70]Fullerene
[60]fullerene derivatives from ultrasonication, however, we found differences among the three structural isomers of the mannosyl [60]fullerene derivatives to the chemical shifts of the N-methylene carbon atom; the N-methylene carbon atom of the open [5,6]-bridged and the closed [6,6]-bridged isomers appeared at 51.57 and 49.91 ppm, respectively, while this carbon for the closed [5,6]-bridged isomer resonated at 47.94 ppm [17,18]. Furthermore, in the case of the mannosyl[70]fullerene derivatives from the ultrasonication, the Nmethylene carbon of the major adduct in the mannosyl [70]fullerene derivatives resonated at 47.85 ppm, similar to that reported for the closed [5,6]-bridged isomer of the mannosyl [60]fullerene derivatives [18,19]. Therefore, these values of the monoadducts enabled us to elucidate the structure of the major adduct in 2, and these results strongly suggest that the major adduct in 2 is the closed [5,6]-bridged isomer. Moreover, ultrasonication promoted reaction of C70 with methyl azidoacetate (1) leading to only a monoaducts, while the thermal reaction afforded the mixture of monoadducts and bisadducts. The results of 2 are presented in Table 1.
UV-vis spectroscopy was used to monitor multilayer closed [5,6]-bridged aziridino[70]fullerene derivative-gold nanoparticle films. The data were collected multiple times and fell in the absorbance range of ± 10%. The reactive slides were alternately immersed in the toluene solution containing closed [5,6]-bridged aziridino[70]fullerene derivative (10 mM) and 4-aminothiophenoxide/hexanethiolate protected gold nanoparticles (~14 μM) for indicated period. UV-vis spectra of nanoparticle multilayer films showed that the surface plasmon (SP) band of gold at 527 nm gradually became more evident as successive layers were added to the films. This enhancement (and shift from~520 nm) suggested that nanoparticle cores were induced to approach one another through interactions between closed [5,6]-bridged aziridino[70]fullerene derivative and amine moieties (Fig. 3). The results also showed that the longer the immersion time, the stronger the absorbance of UV-vis spectra. This suggests alternately the continuous adsorption of closed [5,6]-bridged aziridino[70]fullerene derivative and functionalized gold nanoparticles due to the amination of closed [5,6]-bridged aziridino[70]fullerene derivative.
Cycloadditive reaction of [70]fullerene with methyl azidoacetate (1) at 25~43℃ was promoted by ultrasonication
to give the 1:1 methyl acetate-[70]fullerene adduct 2. FABMS and 1H-NMR spectra revealed that the product 2 was a mixture composed of three different types of monoadducts with identical masses. The analysis of the 13C-NMR and UVvis spectroscopic data indicated that the major adduct in 2 is the closed [5,6]-bridged isomer. Although the product was obtained in low yield (9.1%), an ultrasonication can be a simple method under mild condition.
The closed [5,6]-bridged aziridino[70]fullerene derivativegold nanoparticle films were prepared using the layer-bylayer assembly method. UV-vis spectra of nanoparticle multilayer films showed that the surface plasmon (SP) band of gold at 527 nm gradually became more evident as successive layers were added to the films. This plasmon band enhancement (and shift from ~520 nm) suggested that nanoparticle cores were induced to approach one another through interactions between closed [5,6]-bridged aziridino[70]fullerene derivative and amine moieties.
![UV-vis spectra of 2 and [70]fullerene.](http://oak.go.kr/repository/journal/10462/HGTSB6_2009_v10n4_325_f001.jpg)
![Characterization of Reaction Product between Methyl Azidoacetate (1) and [70]Fullerene](http://oak.go.kr/repository/journal/10462/HGTSB6_2009_v10n4_325_t001.jpg)
![UV-vis absorption spectrum of the layer-by-layer assemblies the closed [56]-bridged aziridino [70]fullerene derivativegold nanoparticle multilayer films for the indicated time; +24 h +24 h +24 h +24 h +24 h (from bottom to top).](http://oak.go.kr/repository/journal/10462/HGTSB6_2009_v10n4_325_f003.jpg)