Electrochemical impedance spectroscopy (EIS) is an important tool in the field of chemistry, including electrochemistry, biochemistry and interfacial science. In particular, EIS is essential for revealing electrochemical phenomena such as the conducting behavior of polymer electrolytes, the interfacial behavior of the solid electrolyte interphase (SEI) on the electrode during the initial charge, and the characteristics of active materials with respect to repeated cycling [1-15]. However, careless handling of the electrode and cell components can cause significantly different EIS spectra for Li-ion secondary batteries [8-12]. Noticeable EIS noise of the active materials used in Li secondary batteries can arise when the counter electrodes are not appropriate. A good counter electrode should remain unchanged or inactive throughout an experiment because impedance signals from counter electrodes are fundamentally included in the measured results. In fact, it has been suggested that Li metal is very sensitive to an organic electrolyte and the sensitive SEI when it arises on its surface, especially showing an explicit EIS spectra divided into semi-circles and lines [5,8,9]. Li metal does not appear to be feasible for use as a counter electrode, though it has been accepted as the best choice for a counter electrode in half-cells for use in material development. The problem with Li metal is that it gives unpredictable signals with respect to the conditions, including the potential and type of surface film used. Along this line, the intent of this study is to check the electrochemical signal noise from Li metal quantitatively and then to remove the noise from a Li counter electrode by introducing an alternative counter electrode.
The key challenge for noise removal in this study is to introduce the same electrode as the working electrode as a half-cell. SEI formation on graphitic carbon before severe Li+ intercalation was also investigated to show the advantages of doing this.
For these experiments, Timrex SFG6 synthetic graphite (Timcal, Switzerland) was selected as the electrode material for Li intercalation behavior. It delivered an initial capacity of ca. 360 mAh g-1 and a high degree of graphitization, an expressed as Lc >100 nm with a (d002)avg value of
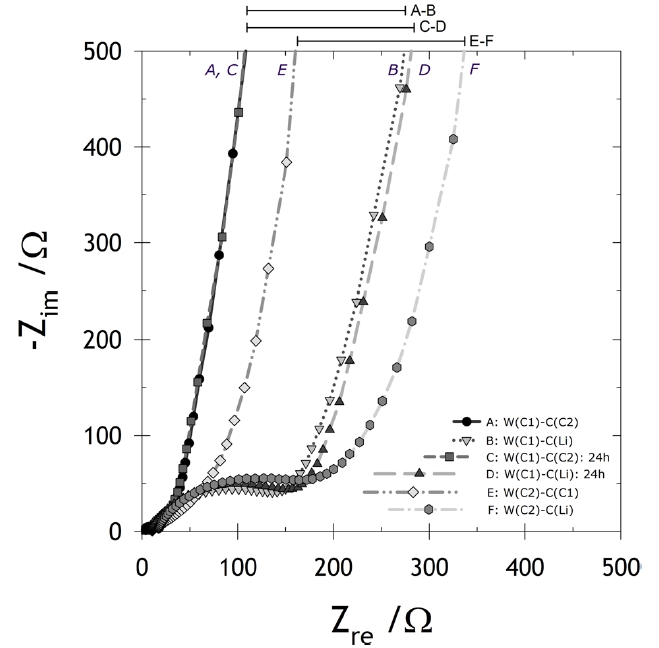
Fig. 2 shows the EIS spectra with respect to the cell configurations labeled as A, B, C, D, E and F, as described in detail in Fig. 2. Two of these schemes (A, B) are not different except for the counter electrodes (C1 in the case of A and Li in the case of B). B showed an additional depressed semi-circle compared to A. This type of semi-circle was likely due to SEI arising on the Li metal [4,8,9]. C2 was discharged against the Li counter electrode (the fourth electrode from the left in Fig.1 a) to 0.8 V vs. Li/Li+ and then stored immediately for 24 h at 25℃. Additional experiments were conducted using C1 (Schemes C and D) and C2 (schemes E and F) as the working electrode, respectively.
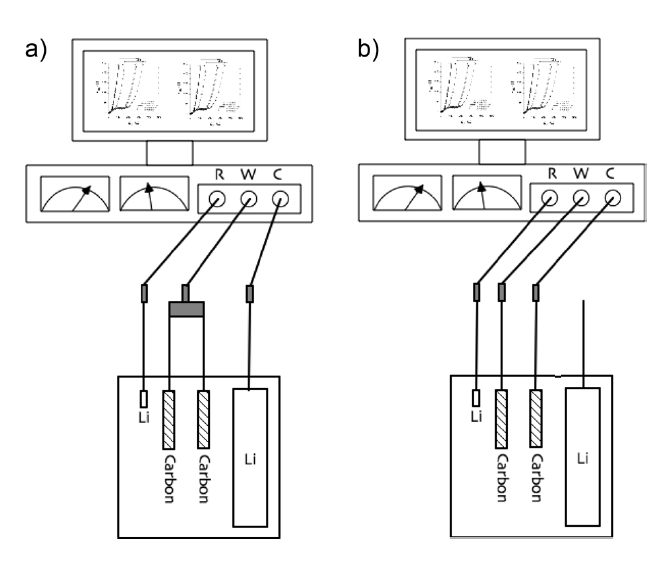
In these cases, semi-circles in B, D and F were observed, serving as direct evidence of the incorporation of the signal from the counter electrode. The graphitic electrode has its impedance signal expressed as a semi-circle, which also indicates SEI on the electrode, if the semi-circles of the working carbon (W(C1) and W(C2)) and the Li counter electrodes (C(Li)) are coupled and if it is difficult and even impossible to decouple the signal of the counter electrode from the total EIS signal. Scheme D may also apply for a typically coupled EIS spectrum consisting of two semi-circles originating from both working and counter electrodes. In this study, we suggest that an electrochemical cell with one switchable electrode can be used to obtain the EIS spectra while eliminated the Li counter noise. This design consists of the SFG6 working electrode, the switchable SFG6 electrode, and two Li electrodes, as described at Fig. 1. This design avoids cell disassembly because the surface of the working electrode in the disassembled cell can be changed easily. As shown in Fig. 1, the SFG6 working electrode and the switchable SFG6 electrode were short-circuited by means of an outer metallic line while the specific potential was adjusted (Fig.1 a). After the removal of the outer metallic line shortly after the voltage adjustment, they were finally used as the working and counter electrodes, respectively, for the EIS measurement (Fig.1 b). The working and switchable SFG6 electrodes delivered equivalent electrochemical conditions, including the electrode potentials, in all experiment times.
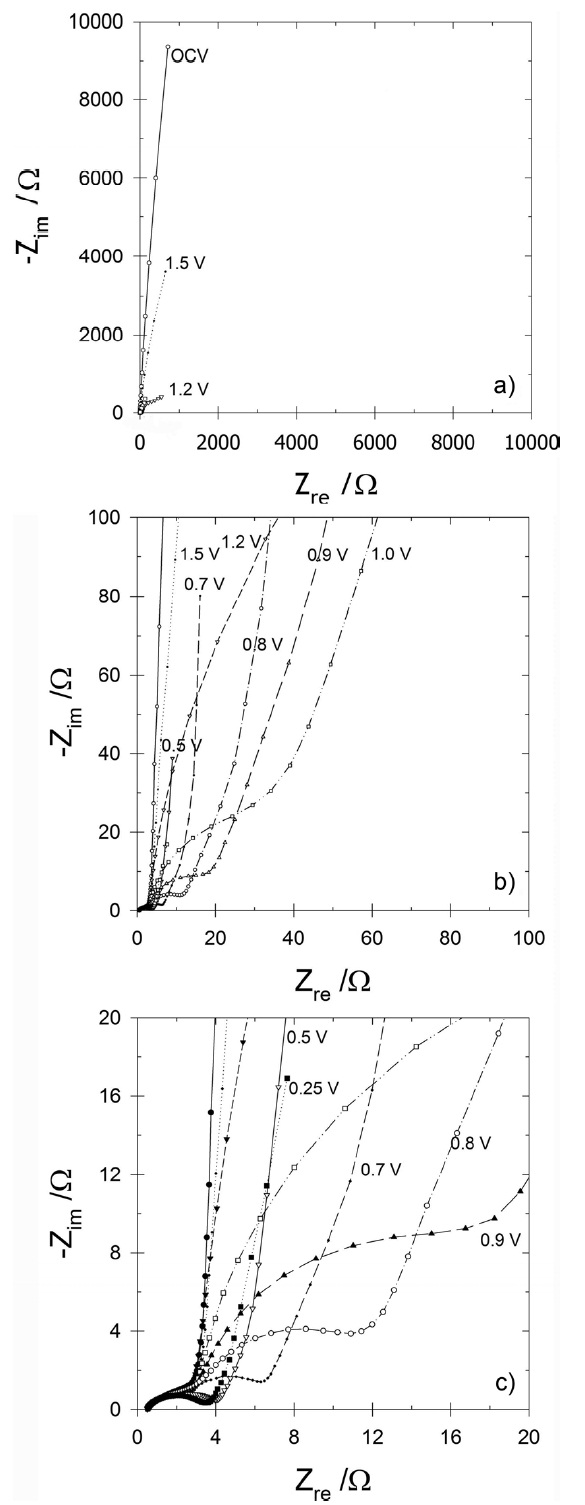
To check the feasibility of using an electrochemical cell with the switchable SFG6 electrode, the electrochemical behavior of graphitic carbon during initial intercalation was investigated considering the electrochemical characteristics of the SEI that had formed in earlier studies [8,10,11,14,15]. Fig. 3 shows the
EIS spectra measured at from the open circuit voltage (OCV,
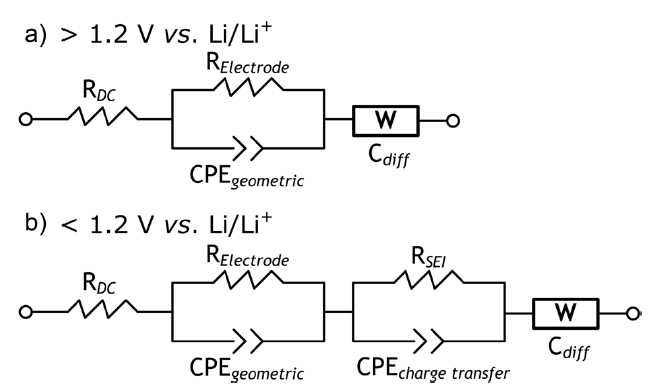
are defined in the caption of Fig. 4. The selected potential points for obtaining the EIS spectra were the OCV, 1.5, 1.2, 1.0, 0.9, 0.8, 0.7, 0.5 and 0.25 V. The EIS spectra of the graphitic carbon in Fig.3 c can be categorized simply as i) direct current (DC) resistance, ii) a potential-insensitive semi-circle in the high-frequency realm, iii) a potential-sensitive converged semi-circle, denoting the region in the mid-frequency realm, and iv) a linear region in the low-frequency realm. The DC resistance showed the same value in all of the selected potentials, reflecting the conductivity of the electrolyte used (1 M LiPF6/EC+DEC). The semi-circle in the high-frequency realm was observed even at the OCV and was insensitive to all selected potentials. There was no Li+ population in or transfer into the carbon at the OCV. This likely represents geometric capacitance (a sort of constant phase element) and physical resistance depending on the physical structure of the electrode. Regarding the potential-sensitive semi-circle in the mid-frequency realm, it changed consistently with respect to the potential. Another capacitive-like element started to appear slightly at ca. 1.2 V and then clearly at ca. 0.5 V, where the newly formed semicircular region most likely indicated Li+ residence and transfer in the SEI generated in this potential region. It should be noted that the semi-circle in the mid-frequency realm at 0.5-1.2 V was quite different from that of scheme C with the Li counter electrode, as shown in Fig. 2, which originated from another instance of SEI on the Li metal. This has been reported frequently in the case of Li working electrodes[8,14,15]. This potential-sensitive semi-circle in the midfrequency realm can provide important information regarding the behavior of Li+ transfer through and during the SEI. When formulating Li+ transfer and residence through SEI with respect to the potential, resistance against Li+ intercalation was lowered until its values were similar to those at a potential of less than 0.5 V. Because the SEI showed both capacitive and resistive behavior, as shown in Fig. 3 c, if describing SEI geometrically, the semi-circle type of signal from the SEI can be conceived of as an interfacial region, including a slightly exfoliated surface. The line appeared in the low-frequency realm (Fig. 3 a) likely indicates Li+ finite length diffusion throughout the graphitic lattice; the end point of the line was found to be proportional inversely to the differential limiting capacitance (Cdiff) [8]. This line is very similar to those obtained using a specially prepared counter Li electrode [8,14,15].
EIS spectra with only the information of the working electrode material could be acquired using an electrochemical cell with an additional switchable electrode upon an energy-related material investigation. Based on our results, we note that Li metal is not feasible as a counter electrode when searching for an active material for Li secondary batteries. A switchable electrode equivalent to the working electrode is proposed as an alternative to a Li-metal electrode. If this is tested, data never previously obtained can be acquired, and this data will provide new information about energy storage materials such as Li ion batteries and electrochemical capacitors.