



All over the world, coal is a major energy resource which is mainly burnt to produce electricity.It is not known to be a clean fuel. Various types of pollutants, such as SOx, NOx and mercury, are released into the atmosphere when coal is burned. Mercury emissions have attracted an increasing amount of concern due to the high toxicity, volatility, bioaccumulation in the environment and the neurological effects of mercury. According to the Global Mercury Assessment Report, coal-fired power plants are the primary source of anthropogenic emissions of mercury into the atmosphere [1-14].
In particular, mercury is one of the most hazardous air pollutants due to its neurological toxicity, volatility, persistence, and bioaccumulation, all of which pose a great threat to both human health and organism security [15-18]. Among human activities, coal combustion makes the greatest contribution as an anthropogenic source of mercury emission. Coal-fired flue gas can contain three forms of mercury: elemental mercury (Hg0), oxidized mercury (Hg2+) and particle-bound-mercury (HgP) [19,20]. During coal combustion, elemental mercury is released and partly oxidized to Hg2+ in the flue gas. The adsorption of Hg0 and Hg2+ on solid surfaces leads to the formation of the particle-bound mercury. Particle-bound mercury (HgP) can be easily removed by dust collection processes. Furthermore, oxidized mercury is soluble in water and easily adsorbed on solid surfaces, which aids its removal [21]. However, Hg0 is neither soluble in water nor easily adsorbed. It has a lifetime of 1-2 years in the atmosphere and can be transported over long distances to cause widespread mercury pollution. Therefore, the removal of Hg0 emitted from flue gas is an important part of minimizing mercury emissions [22].
Many technologies have been implemented to control Hg0 emissions, such as catalytic or photochemistry oxidation, sorbent injection and air pollution control devices (APCDs). Adsorption using porous carbon (PC), particularly PC impregnated with sulfur (S), chlorine(Cl), or iodine (I), has excellent potential for Hg0 removal from flue gases.
In this paper, we review the processing methods and the various materials used for the removal of elemental mercury.
Lee et al. [23] reported commercial brominated activated-
carbon (DARCO Hg-LH) and cupric chloride-impregnated activated carbon (C-AC) that were tested in fixed-bed systems. Fig. 1 shows the outlet elemental mercury (Hg0) and oxidized mercury (Hg2+) concentrations as measured for each of the 12-h fixed-bed tests of DARCO Hg-LH and C-AC. In the DARCO Hg-LH and C-AC, very slight amounts of outlet Hg0 (<3% of the inlet Hg0) and Hg2+ (<1% of the inlet Hg0) were found during the first 24 h. After the first 24 h, more outlet-oxidized mercury was found from the test of C-AC than from that of DARCO Hg-LH.After 48 h, the sum of the outlet concentrations of Hg0 and Hg2+ reached the inlet Hg0 concentration, indicating that Hg adsorption onto C-AC was essentially complete.
Zeng et al. [24] studied the removal capabilities of elemental mercury from coal combustion flue gas of chloride-impregnated activated carbon. The experiment results showed that impregnation with ZnCl2 significantly enhanced the adsorptive capacity for mercury vapor but decreased the specific surface area of the activated carbon (Fig.2 ).
Hu et al. [25] investigated oxidative adsorption of elemental mercury by activated carbon in simulated coal-fired flue gas. Fig. 3 shows the Hg0 adsorption of chlorine-impregnated activated carbon. The adsorption of Hg0 by activated carbon was a complete chemical adsorption process in N2 gas or in simulated
flue gas. Hg0 was oxidized to Hg2+ by chlorine on the carbon surface and absorbed by activated carbon in N2 gas. The oxidizing elements were consumed during the adsorption process.
Qiao et al. [26] reported the adsorption and catalytic oxidation of gaseous elemental mercury in flue gas over MnOx/alumina.MnOx/Al2O3 showed a significant adsorption capability to capture Hg0 in the absence of hydrogen chloride (HCl). The ideal adsorption temperature was about 600 K (Fig.4 ).
Mercury removal with catalytic oxidation, particularly when used in conjunction with sulfur (S), chloride (Cl), or iodine (I) impregnation, has also been studied for the removal of Hg0 from flue gas. Recently, catalysts employing manganese oxides as the active ingredient have attracted substantial attention in the selective catalytic reduction field owing to their high catalytic activity in a wide range of temperatures.
Photochemistry oxidation of mercury with various components in flue gas may be an attractive alternative to sorbent [15,27,28] or scrubber-based [29] processes for mercury capture applications. One widely studied area and relevant has been the photochemistry oxidation of mercury using 253.7 nm ultraviolet light [30-37]. Dickinson and Sherrill [30] demonstrated the photochemical formation of mercuric oxide via the sensitized formation of ozone in 1926. The mechanism between mercury and oxygen in the presence of 253.7 nm radiation is expressed below as Eq. (1).
In the reaction mechanism, elemental mercury serves as a sensitizer for the formation of ozone, and the ozone oxidizes mercury to form mercuric oxide [30]. Photochemistry oxidation is a potential means of removing mercury from flue gas. The photochemical formation of mercuric oxide can also have a significant impact on current ultraviolet-based methods of measuring mercury in flue gas as well as potential environmental
[Table 1.] Photochemical removal of mercury from flue gasesa [29]
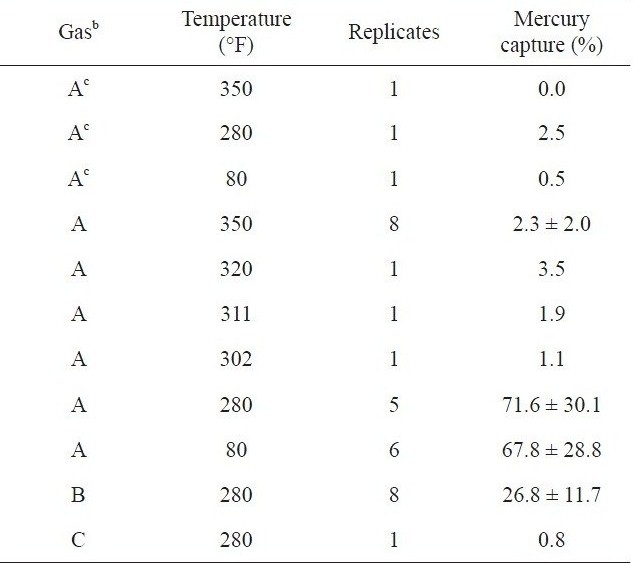
Photochemical removal of mercury from flue gasesa [29]
[Table 2.] Effect of radiation intensity on mercury removala [29]
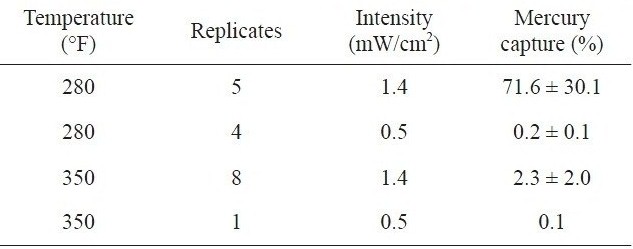
Effect of radiation intensity on mercury removala [29]
consequences [31].
Granite and Pennline [29] found that photochemical reactions of mercury with various constituents in flue gas produced by burning coal could be an attractive alternative to dry sorbent or wet scrubber-based processes for mercury control. Mercurycontaining simulated flue gases at temperatures between 80 and 350 ℉ were irradiated with 253.7 nm ultraviolet light. The elemental mercury adsorption increased by about 28 times at 280 ℉ (a gas condition), as shown in Table .1 A decrease in the radiation intensity by a factor of about 3 causes a much larger decrease in the mercury capturing capacity (Table 2).
Woo et al. [38] studied a PCO process for the photochemical removal of mercury from flue gas. The preliminary tests conducted to date clearly show the ability of the PCO process to oxidize elemental mercury, as shown in Figs. 5 and 6. Similar results were observed by Pitoniak et al. [39] for silicatitania nanocomposites for elemental mercury vapor removal (Fig.7 ).
Jeon et al. [6] researched the elemental mercury removal of TiO2 powder under various light sources, including UV light
(black and sterilizing, >99%), fluorescent light (home-style, >99%), and visible blue light (>400 nm, ~85%). These studies showed that a far stronger bond formed between mercury and TiO2 under photochemistry oxidation. For elemental mercury photo-oxidation with TiO2, a mechanistic illustration was given [40,41].
Photochemistry oxidation is possible under UV light for the measurement of mercury. Recently, this process of elemental mercury removal has been studied under various light sources.
Mercury control via the injection of sorbent materials into the gas stream of coal-fired boilers is under development. Currently,this approach is being demonstrated on selected full-scale systems. A typical implementation of this control technology would entail the injection of powdered sorbent upstream of a particulate matter (PM) control device (electrostatic precipitator [ESP] or fabric filter [FF]). An alternative is the TOXECON configuration, in which a relatively small FF is installed downstream of an existing ESP. Sorbent is injected downstream of the ESP after most of the flue-gas PM has been removed. The sorbent is then collected in the downstream FF, which effectively segregates the fly ash and injected sorbent.
Some of the factors that appear to affect the performance of any particular sorbent include the method and rate of sorbent injection; the flue gas conditions, including the temperature and concentrations of the halogen species (e.g., HCl) and sulfur trioxide (SO3); the existing APC configuration; and the physicochemical characteristics of the sorbent. The sorbent injection rate is usually expressed as pounds of sorbent per million actual cubic feet of flue gas (lb/MMacf). For a 500-MW boiler, a sorbent rate of 1.0 lb/MMacf corresponds to ~120 lb/h of sorbent [42].
Carey et al. [43] investigated the effectiveness of sorbent injection as a means of mercury control in flue gas streams. Mercury adsorption tests conducted at two different utility sites indicate that the sorbent characteristics are dependent on the flue gas conditions. Based on the sorbent characteristics measured at two field sites using a commercially available form of activated carbon, the predicted carbon injection rates to achieve 80% mercury removal can differ by a factor of 2-5, as shown in Figs. 8 and 9.
Morency [44] reported a zeolite sorbent method that effectively removes mercury from flue gases. The zeolite sorbent and activated carbon were injected into coal combustion flue gases in a laboratory reactor. The Hg capture results were enhanced 100% using a treated zeolite: Hg ratio of 25 000, as shown Fig.10.
Kim et al. [45] and Park and Seo [46] studied the elemental mercury adsorption behavior of chemically modified activated carbon. Fig. 11 shows that the elemental mercury adsorption characteristics of the acid-treated activated carbon were found to be three to four times better than those of non-treated activated carbon or base-treated activated carbon, respectively.
Fig. 12 from our previous research [47-49] shows various pore structures prepared in an optimized manner for mercury vapor adsorption. Micropore materials (ACs) showed a high elemental vapor adsorption rating of 295.2 μg/m3. However, comparing two types of mesopore materials (SBA-15 and MCM-41), the mercury vapor adsorption was higher in SBA-15, as SBA-15 has a higher micropore volume fraction than MCM-41.We can conclude that mercury vapor adsorptions rates can be optimized in terms of the specific surface area and micropore fraction [47]. Fig. 13 shows the elemental mercury adsorption behavior of Cu/PCs. The elemental mercury adsorption of all Cu-ACs occurred at a level higher than that of the as-received sample. The efficiency increased as the plating time increased to Cu-15 and then decreased with Cu-25 despite the similar specific surface areas and total pore volumes. In conclusion, there is a strong correlation between the Cu2O/Cu ratio and the mercury-removal properties of Cu-coated porous carbonaceous materials [48]. Fig. 14 shows the elemental mercury adsorption characteristics of metal/activated carbon hybrid materials. Based on the experimental results, elemental mercury adsorption of all metal/ACs occurred at a level higher than noted with the as-received sample. This
demonstrates that metal plating (Cu and Ni) on carbon surfaces can be a feasible method of elemental mercury adsorption [49].
2.4 Air pollution control devices
Mercury species in coal-fired flue gas include elemental, oxidized and particulate-bound mercury. The most common APCDs in US coal-fired utility power stations include (1) ESP used with or without flue-gas desulfurization (FGD) controls (Fig.15 ), and (2) FF, which may be used alone or with spray dry absorbers (SDAs). These control devices are designed to remove particulates (FF, ESP) or S (FGD, SDA) from flue gases. The position of an ESP relative to the air pre-heater device, down-stream of the boiler, is an important distinction. A cold-side ESP is installed downstream of the air pre-heater (Fig.16 ), whereas a hot-side ESP is installed upstream of the air pre-heater, closer to the boiler. The amount of Hg removed by an APCD depends on its type and on the rank of coal being burned. Empirical results [50] show the most efficient level of Hg removal for bituminous coal (>60%) in FF/SDA and FGD devices (Fig.17 ). In contrast, a cold-side ESP (CESP) removes only 40% of Hg from flue gases, while a hot-side ESP (HESP) has virtually no effect on the pollutant. At plants burning subbituminous coal or lignite,
APCDs are much less capable of removing Hg. For the former fuel, only FFs (60%) and FGD systems (15%) have shown good effectiveness. In the case of lignite-burning plants, the removal of Hg is very low, in some cases apparently negative. Mercury removal is calculated from measurement of Hg in the flue gas at the inlet and outlet of the particulate control device. When little Hg is removed, limitations on the accuracy of the inlet and outlet measurements may result in apparent negative removals.Equations describing the Hg removal behavior of these devices as a function of coal quality parameters have been fit to the ICR
data; these results were compared and evaluated by Quick [51].
Heterogeneous reactions involve adsorption and desorption, leading to the adsorption of gas-phase Hg onto solid particles (Hgp) and the formation of Hg2+. A multi-step heterogeneous model has been proposed by researchers at the University of North Dakota’s Energy and Environmental Research Center to characterize the adsorption and oxidation of Hg by activated C [52]. The active sites are postulated to be C sites that act as Lewis bases. Oxidized Hg in flue gas (Hg2+) can bind to these
sites. Acid gases (HCl, SO2, SO3) also bind to these sites. HCl bound to a C site is postulated to adsorb elemental Hg from the gas; oxidants in the gas (O2, NO2, NO) subsequently oxidize the Hg to Hg2+, which can stay bound to the C or desorb, primarily as HgCl2. Sulfur species (SO2, SO3) compete for the basic sites and can inhibit the binding of Hg to the sites. The adsorption of gas-phase Hg by unburned C and its capture in fly ash is an important process in which Hg is trapped in particulate control devices. Often a consequence of the use of low-NOx burners or low-NOx combustion systems, pulverized-coal boilers burning bituminous coal can produce high levels of unburned C, 5-30 wt% as loss-on-ignition (LOI) components. Mercury has been found to be concentrated in the C-rich fraction of fly ash [53-55]. Bituminous coal-ash produced under CESP conditions exhibits a consistent trend of increasing Hg removal with the unburned C content [56]. This trend suggests that a relationship exists between C in ash and Hg removal for plants burning bituminous coal in pulverized-coal-fired boilers with CESP. This relationship appears to be independent of the Cl content of the coal. Data from a plant with an HESP show uniformly lower Hg removal rates for a given percentage of LOI as compared to data from the other pulverized-coal-fired plants (Fig.18 ). HESP operates at temperatures of 300-400℃, whereas CESP operates at temperatures of 135-175℃. The adsorption of Hg by fly ash or activated C has been observed to decrease as the temperature increases [57,58]. The higher temperatures for HESP favor lower adsorption of Hg upstream of the ESP and thus less capturing of particulate-bound Hg in the ESP. Furthermore, the flue gas path between the air pre-heater inlet and the CESP inlet provides additional residence time for Hg sorption, which may contribute to the higher removal of Hg observed for CESP. The Hg removal values for CESP in cyclone and stoker units are much lower than those for pulverized-coal-fired units having similar values of LOI (Fig.18 ). For bituminous-coal fly-ash samples from pulverized-coalfired boilers, the surface area of C present in the ash is between 40 and 100 m2/g C [56], which is in agreement with the values reported by Gao et al. [59] and Pedersen et al. [60] for Class F (bituminous) fly-ash samples. The cyclone- and stoker-ash samples have much lower C surface areas (8-11 m2/g C), which may explain the lower percentage of Hg removal for ESP in
those boilers [56,61]. Data from full-scale combustion systems show that Hg removal for ESPs in pulverized-coal-fired boilers burning bituminous coal appears to be related to the LOI or unburned C in the fly ash. The surface area of the C in the fly ash was remarkably consistent among the samples from pulverized-coal-fired units; combustion systems that produced fly ash with a lower C surface area showed lower Hg removal for all ESP values. These conclusions cannot be extended to fly ash from subbituminous coal and lignite, however. There is evidence in the literature [56,59,61] that the surface area of C from Class C (subbituminous) fly ash samples is significantly higher than that of Class F fly-ash samples on a per gram of C basis.
In this study, we reviewed elemental mercury control technologies (catalytic oxidation, sorbent injection, photochemistry oxidation, and APCDs).
Additional research is needed to identify the mercury compounds that are formed and to verify capture mechanisms. Engineering development is also needed to improve the sorbent dispersion and optimize gas?solid contact time.
![Outlet elemental mercury (Hg0) and oxidized mercury (Hg2+) concentrations measured for each of the 12-h fixed-bed tests of brominated activated carbon (a) and cupric chloride-impregnated activated carbon (b) [23].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f001.jpg)
![Adsorption of elemental mercury onto the as-received and ZnCl2-impregnated activated carbon for a testing time up to 8 h [24].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f002.jpg)
![Hg0 adsorption by activated carbon in N2 and in simulated flue gas [25].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f003.jpg)
![Breakthrough curves of elemental mercury across the catalysts. Air was used as the balance gas and the packed volume of the catalyst was 3.2 mL (corresponding to 3.6 g of MnOx/γ-Al2O3 or 2.4 g of MnOx/γ-Al2O3) if not expressly indicated [26].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f004.jpg)
![Photochemical removal of mercury from flue gasesa [29]](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_t001.jpg)
![Effect of radiation intensity on mercury removala [29]](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_t002.jpg)
![Oxidation of Hg0 with UV light [38].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f005.jpg)
![Oxidation of Hg0 with light [38].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f006.jpg)
![Fractional Hg0 outlet concentration for TiO2-HgO-doped pellets [39].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f007.jpg)
![Comparison of mercury adsorption capacities for sorbents tested in the lab and at site 1 [43].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f008.jpg)
![Comparison of mercury adsorption capacities for sorbents tested in the lab and at site 2 [43].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f009.jpg)
![Hg removal by zeolite sorbent treated by the vapor deposition method [44].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f010.jpg)
![Elemental mercury adsorption characteristics of activated carbons before and after a chemical treatment [45].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f011.jpg)
![Adsorption isotherms of N2/77K (a) and elemental mercury adsorption (b) of activated carbons (ACs) SBA-15 and MCM-41 [47].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f012.jpg)
![Elemental mercury adsorption of metal/activated carbon hybrid materials as a function of the plating time [48].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f013.jpg)
![X-ray diffraction patterns and elemental mercury removal efficiency of the Cu/PC as a function of the plating time [49].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f014.jpg)
![Schematic showing the flow of materials in a coal-fired boiler equipped with an electrostatic precipitator (ESP) and flue-gas desulfurization (FGD) air pollution control devices [61].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f015.jpg)
![Diagram of a coal-fired power plant illustrating the critical pathways for Hg transformation [61].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f016.jpg)
![Average Hg removal across air pollution control devices in coal-fired utility boilers from an Environmental Protection Agency (EPA) Information Collection Request [61].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f017.jpg)
![Mercury removal across coal-fired boilers burning bituminous coal with an electrostatic precipitator (ESP) [56].](http://oak.go.kr/repository/journal/10444/HGTSB6_2011_v12n3_121_f018.jpg)