Water is a precious natural resource vital for sustaining all life on earth. Ever expanding population and their associated activities compounded by increasing rate of overuse, misuse and abuse of limited freshwater sources over the past few decades have imposed threat on sustainable water development and its management.
Synthetic dyes are widely used in many industries including leather tanning, textile dyeing, paper printing etc. Coloured organic substances generally represent only a small fraction of the total organic load in waste water, however, their high degree of colour is easily detectable [1]. Even at low concentrations disposal of these wastes into the environment could be harmful since they reduce light penetration and have a derogatory effect on photosynthesis. In addition, most dyestuffs are designed to be resistant to environmental conditions such as light, heat and microbial attack.
Various techniques, such as chemical coagulation using alum, lime, ferric chloride, ferric sulphate, biosorption [2] oxidation methods using chloride and O3 [3], membrane separation [4], biological treatment, floatation [5] and adsorption have been employed to remove dyes from industrial effluents.
Activated carbon has been successfully employed for removal of colour from aqueous solution [6]. They are most versatile and unique adsorbents because of their high adsorption capacities, high degree of surface reactivity, extendable surface area and micro porous structure [7]. However, commercial activated carbons are still considered expensive[8] and hence not suitable for developing countries. Currently for such countries, the most suitable technology seems to adsorption onto cheap and abundantly available biomass since this is an eco-friendly and economically feasible dye removal technique [9].
From the related literature, several adsorbents have been reported as dye adsorbents. To name a few, materials such as fly ash [10-14], mango seed and shell [15-16], bagasse [17], sawdust [18-21], coconut shell [22, 23], groundnut husk [24], used waste tea leaves [25] etc. have been reported.
The purpose of the present work was to investigate the potentialities of low cost and easily available bio-wastes i.e. cow dung ash, mango stone ash, parthenium leaves ash and activated carbon for the adsorptive removal of Acid Green 20. The effects of some operating parameters (pH, dye concentration) on the biosorption process were investigated. In addition, the applicability of the Langmuir and Freundlich isotherm models were investigated.
Adsorption studies were performed by the batch technique using biomass ash (low cost bio-waste) and activated carbon as the adsorbents without giving any pretreatment. A stock solution of the dye with a concentration of 1000 mg/ L was prepared and dilutions were made with distilled water to make different concentrations (10 ~ 100 mg/L) for the adsorption studies. A known weight of the adsorbent (1 g) was added to 50 mL of each of the above concentration in 100 mL
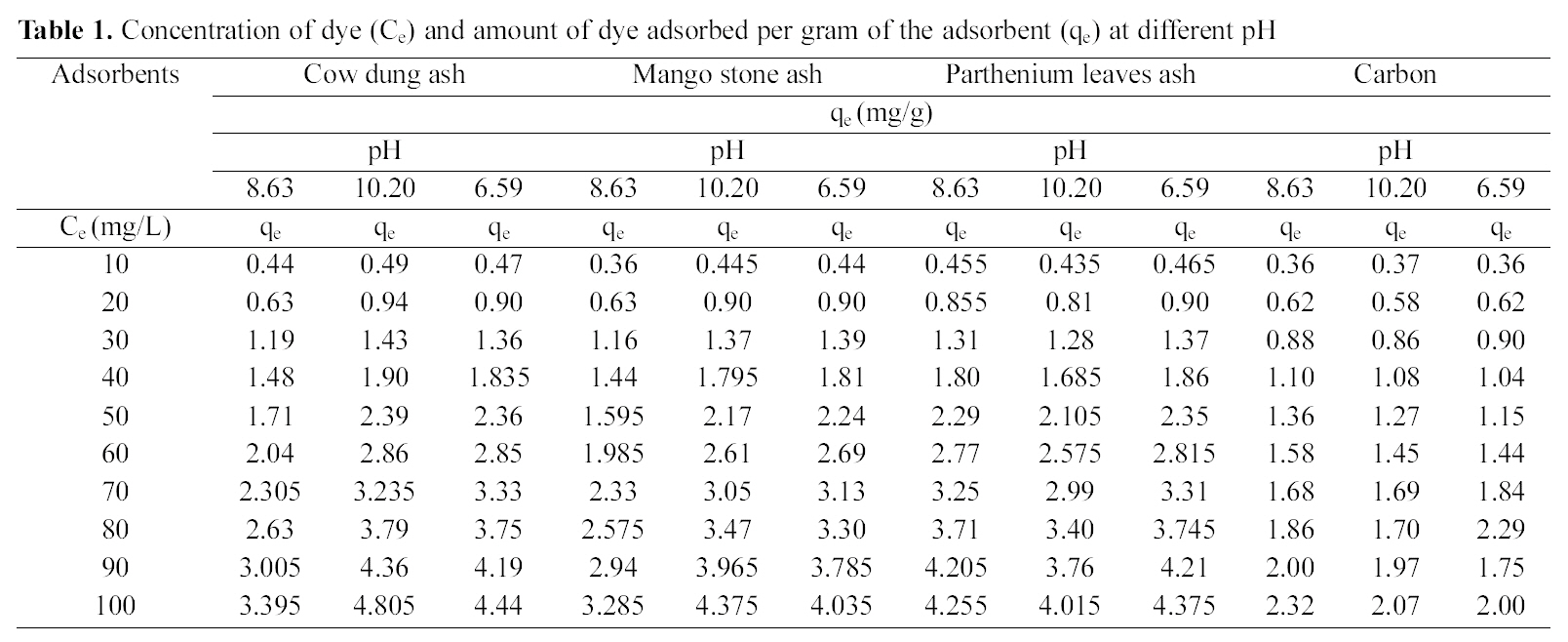
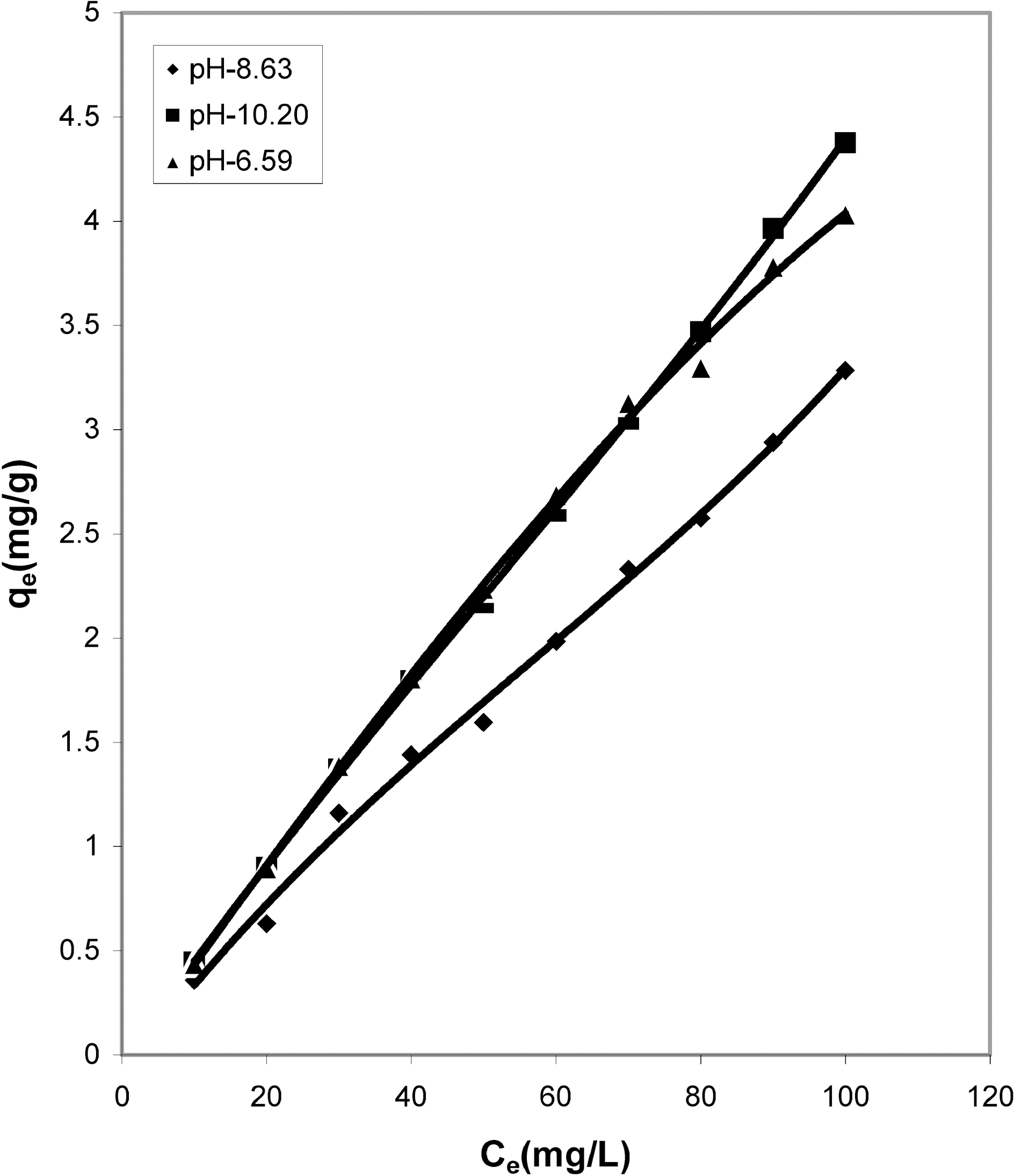
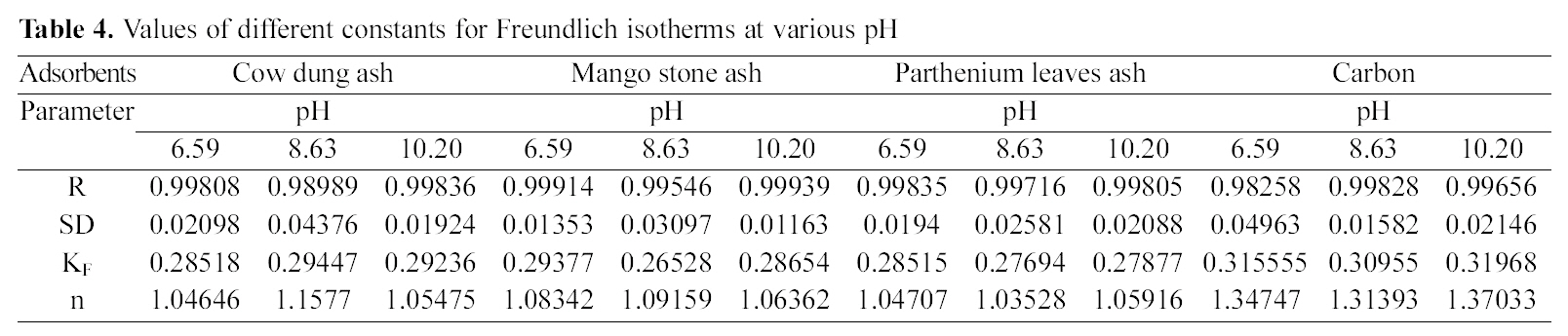
Concentration of dye (Ce) and amount of dye adsorbed per gram of the adsorbent (qe) at different pH
measuring flasks. These were placed in an air thermostat for 24 hrs. with occasional shaking. The samples were then filtered and analyzed using UV-spectrophotometer. Wavelengths of different dyes were determined by λmax method. The pH values of solutions were adjusted by addition of H2SO4 and NaOH.
3.1. Effect of initial dye concentration
Amount of dye adsorbed is tabulated in Table 1 from which it is seen that with a constant adsorbent dose of 1 g/
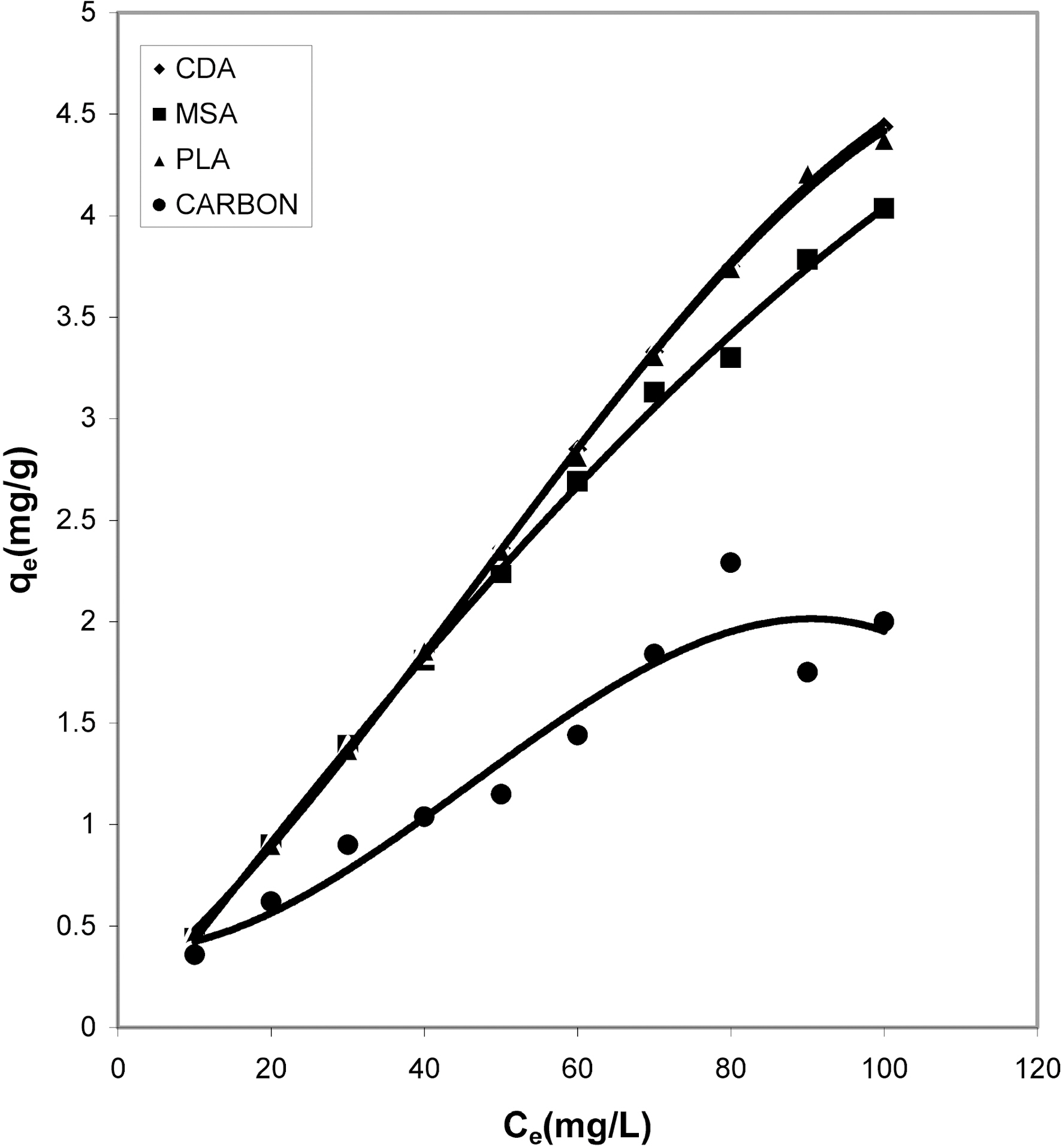
50 ml of dye solution, the amount of dye adsorbed per gram of adsorbents increases with the increase in the dye concentration. The data shows that adsorption on bio-wastes was more than activated carbon and they adsorbed a fairly good amount of residual dye Acid Green 20 from wastewater.
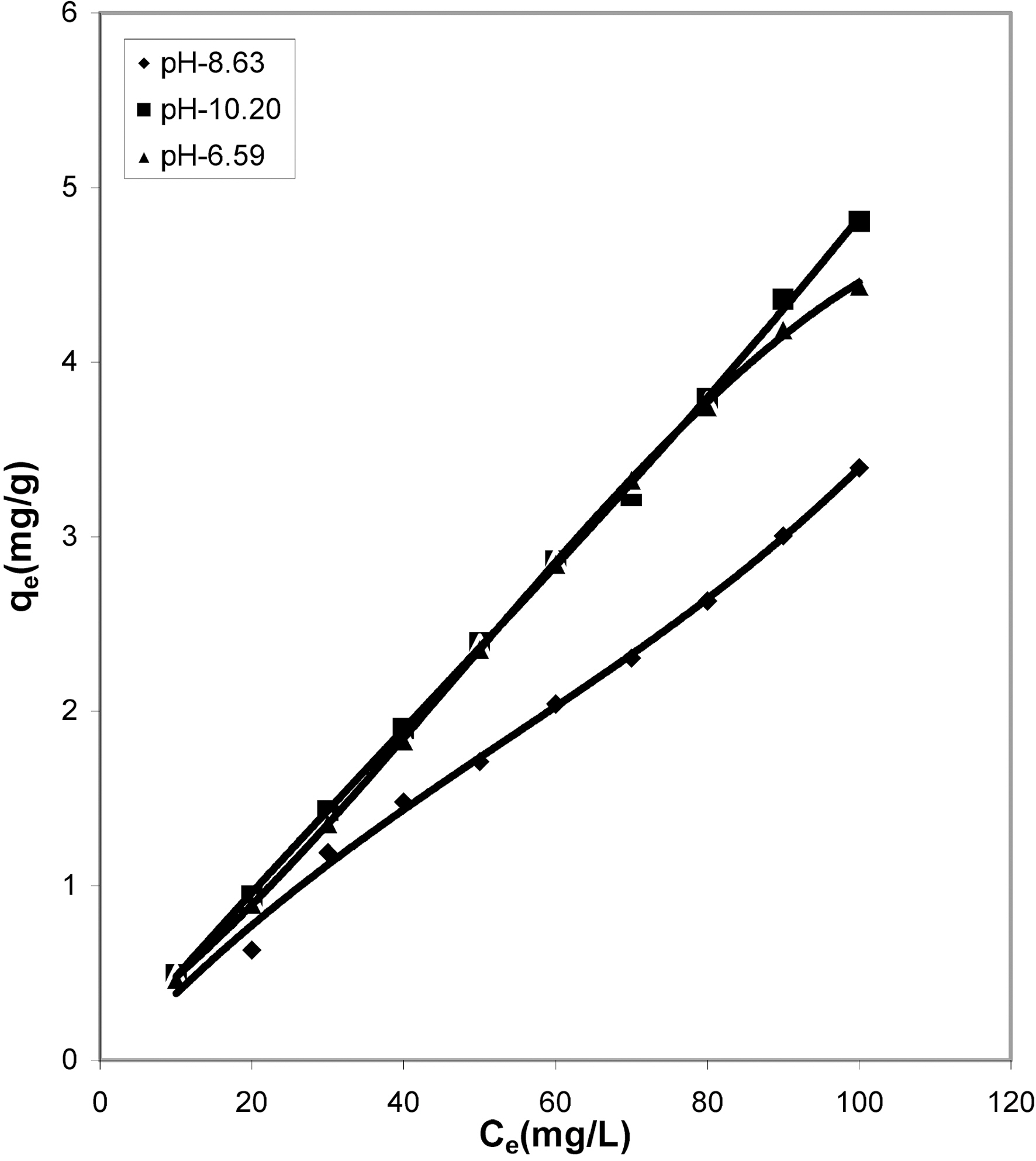
The pH has a marked effect on the adsorption of dyes. At different pH values the amount adsorbed per gram of adsorbent is different. It is seen from Table 1 that cow dung and mango stone ash adsorbed better at strong basic pH i.e. 10.20 (4.805 mg/g and 4.375 mg/g) while adsorption was less
at the other two pH values, 8.63 (3.395 mg/g and 3.285 mg/g) and 6.59 (4.44 mg/g and 4.035 mg/g). For parthenium leaves ash pH 6.59 suited more (4.375 mg/g) while for activated carbon it showed least adsorption capacity (2.0 mg/g).
3.3. Data fit for Simple Isotherms
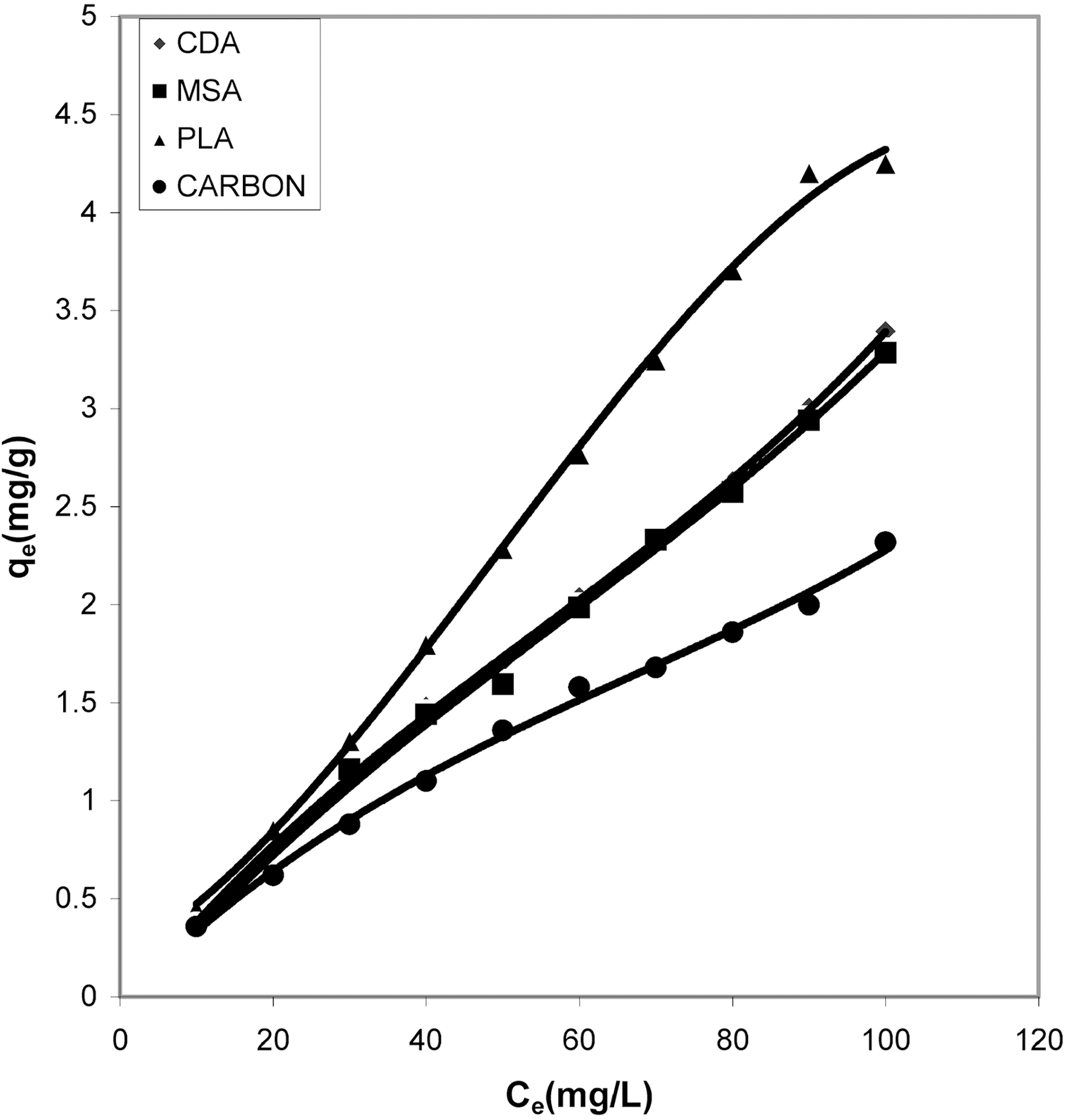
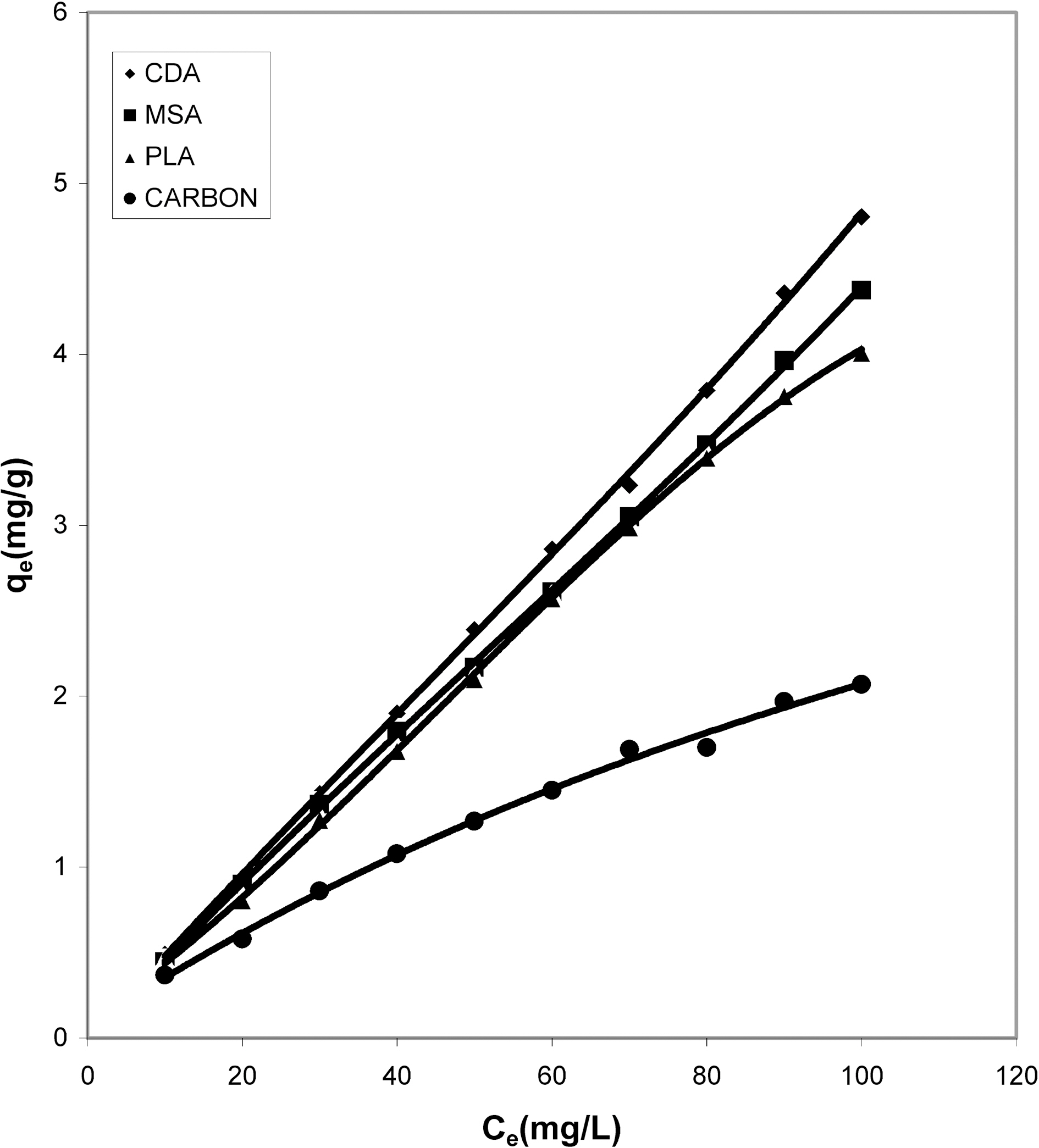
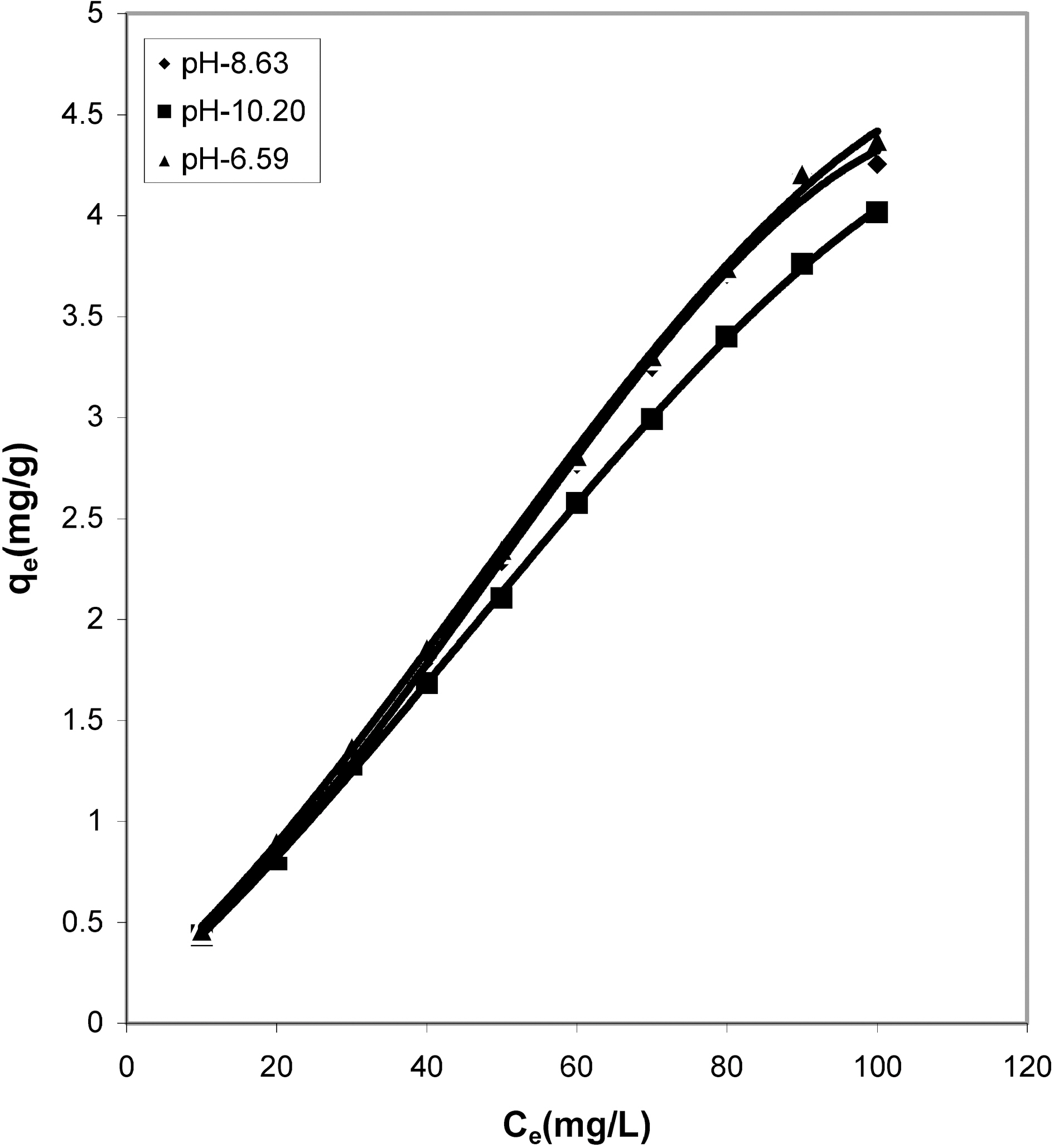
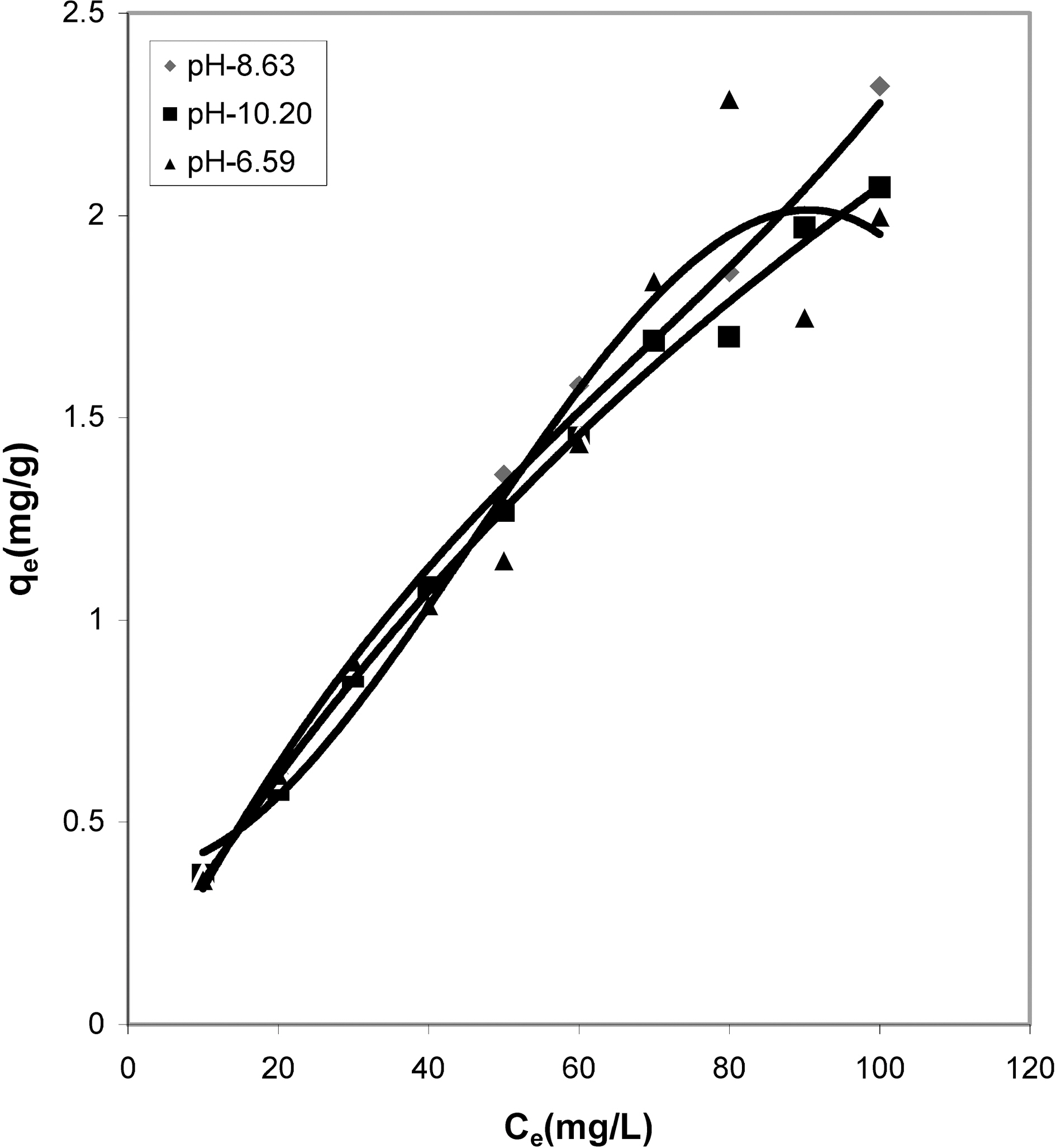
It can be seen from Figs. 1, 4, 7 that the experimental data
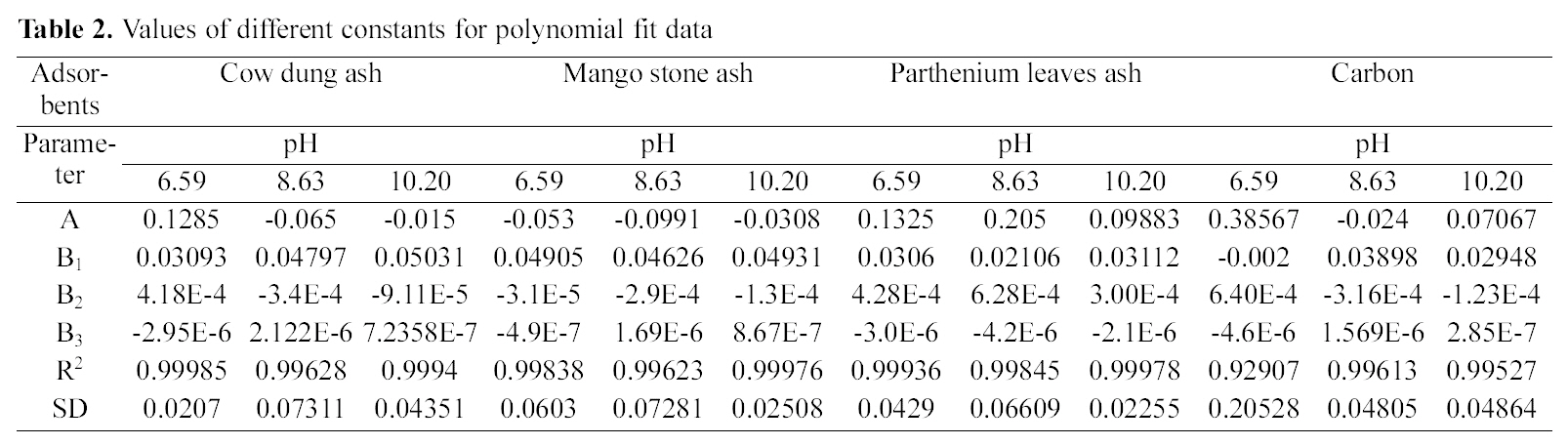
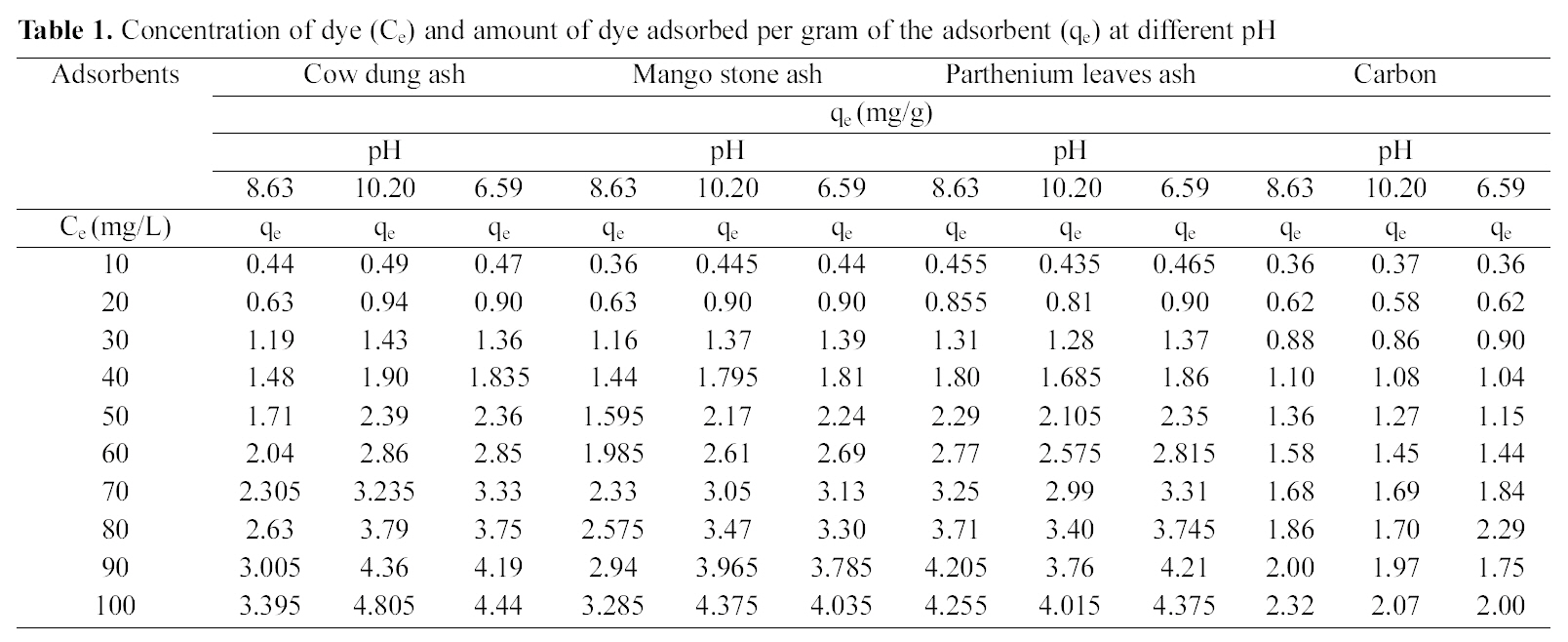
fitted well to the Polynomial Equation 1. The constants and standard deviation are given in Table 2. Comparative adsorption of Acid Green 20 on the various adsorbents used at varied pH values is shown in Figs. 1, 4, 7. Maximum dye removal was observed at pH 10.20. Activated carbon showed the least adsorption efficiency as compared to the biomass ash i.e. cow dung, mango stone and parthenium leaves ash.
Cow dung and mango stone ash (Figs. 10 and 11) exhibit most favorable adsorption under strong alkaline condition
[Table 2.] Values of different constants for polynomial fit data
Values of different constants for polynomial fit data
(pH 10.20) whereas at pH 8.63 adsorption is least. From Fig.12 it is seen that where at pH 10.20 cow dung and mango stone ash show maximum adsorption efficiency, parthenium leaves ash shows the least, the best being at around neutral
pH value of 6.59. At pH 10.20 and 8.63 adsorption trend is almost similar. For activated carbon (Fig. 13) adsorption gets quite linear at pH 8.63 and 10.20 but at pH 6.59 it gives an almost S-shape curve showing least adsorption at the highest
dye concentration.
3.3. Langmuir isotherms at various pH
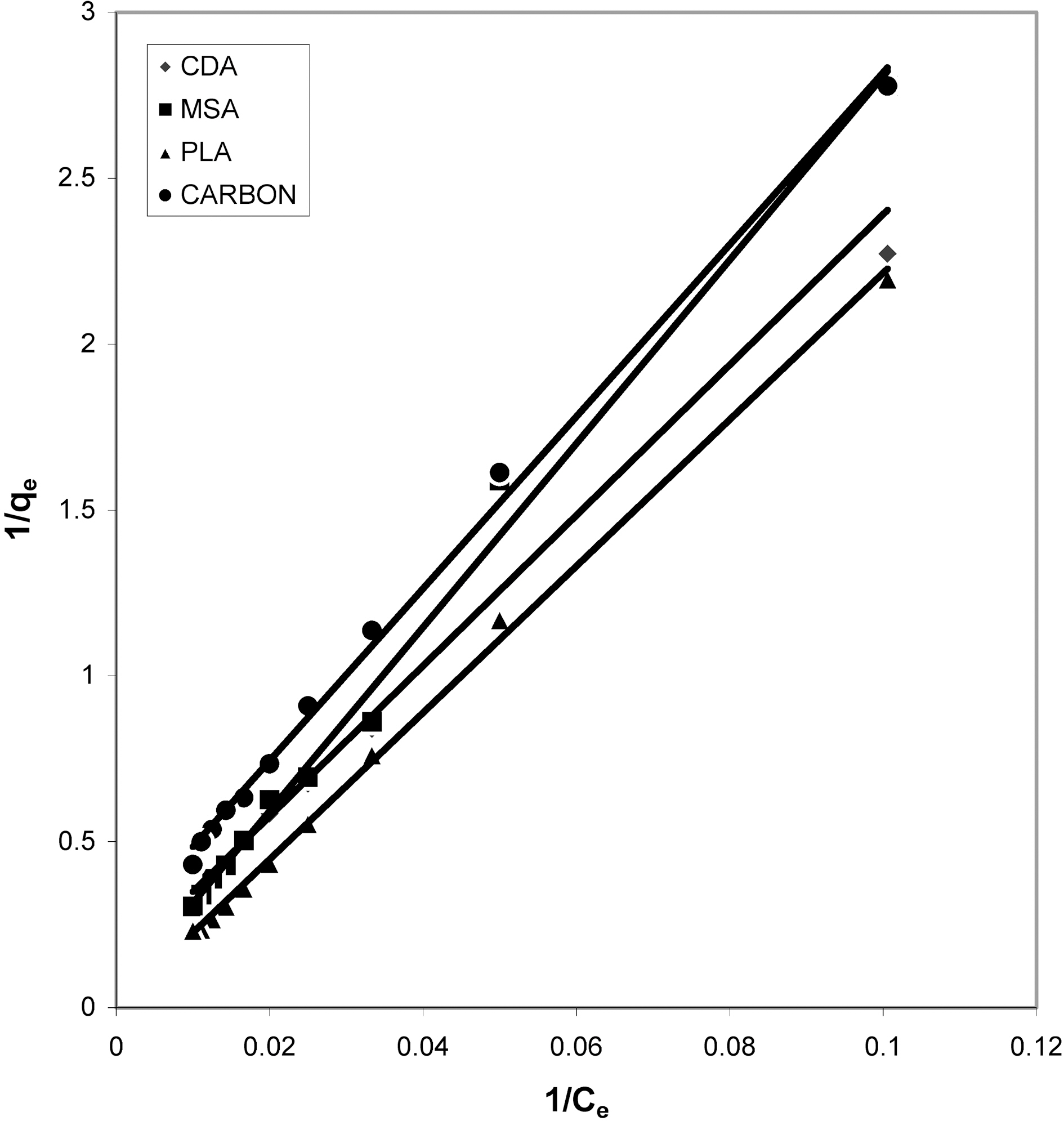
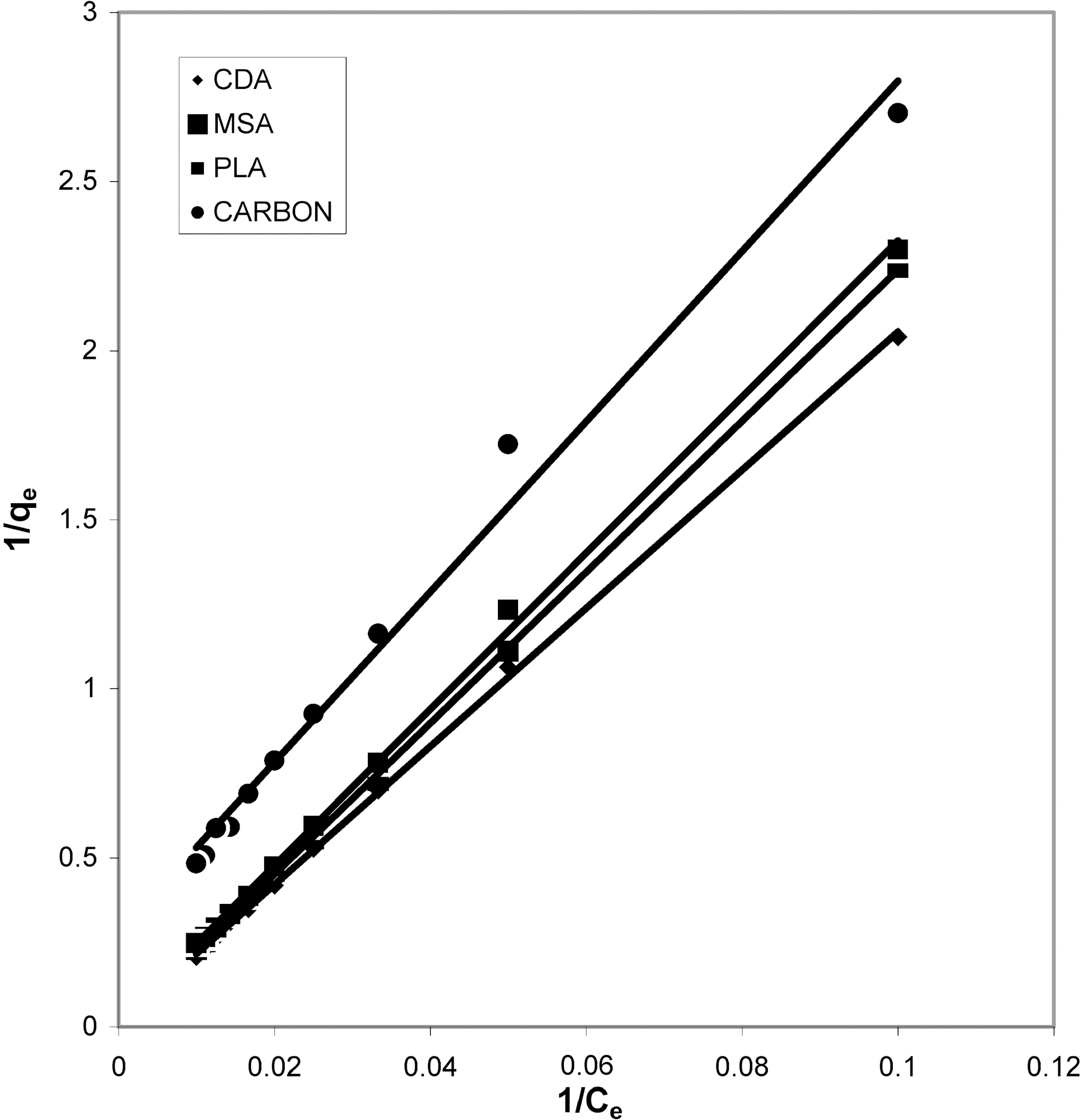
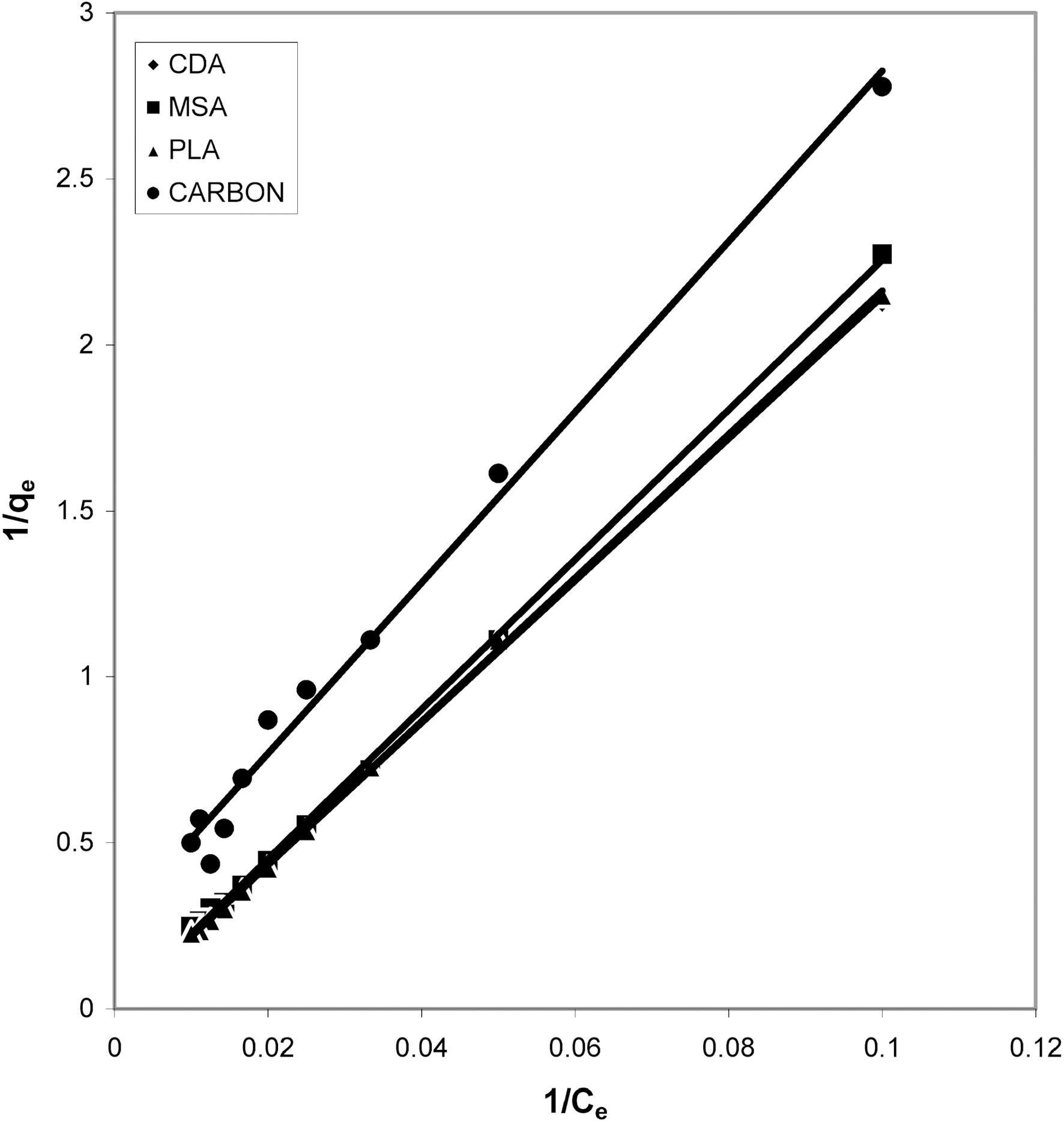
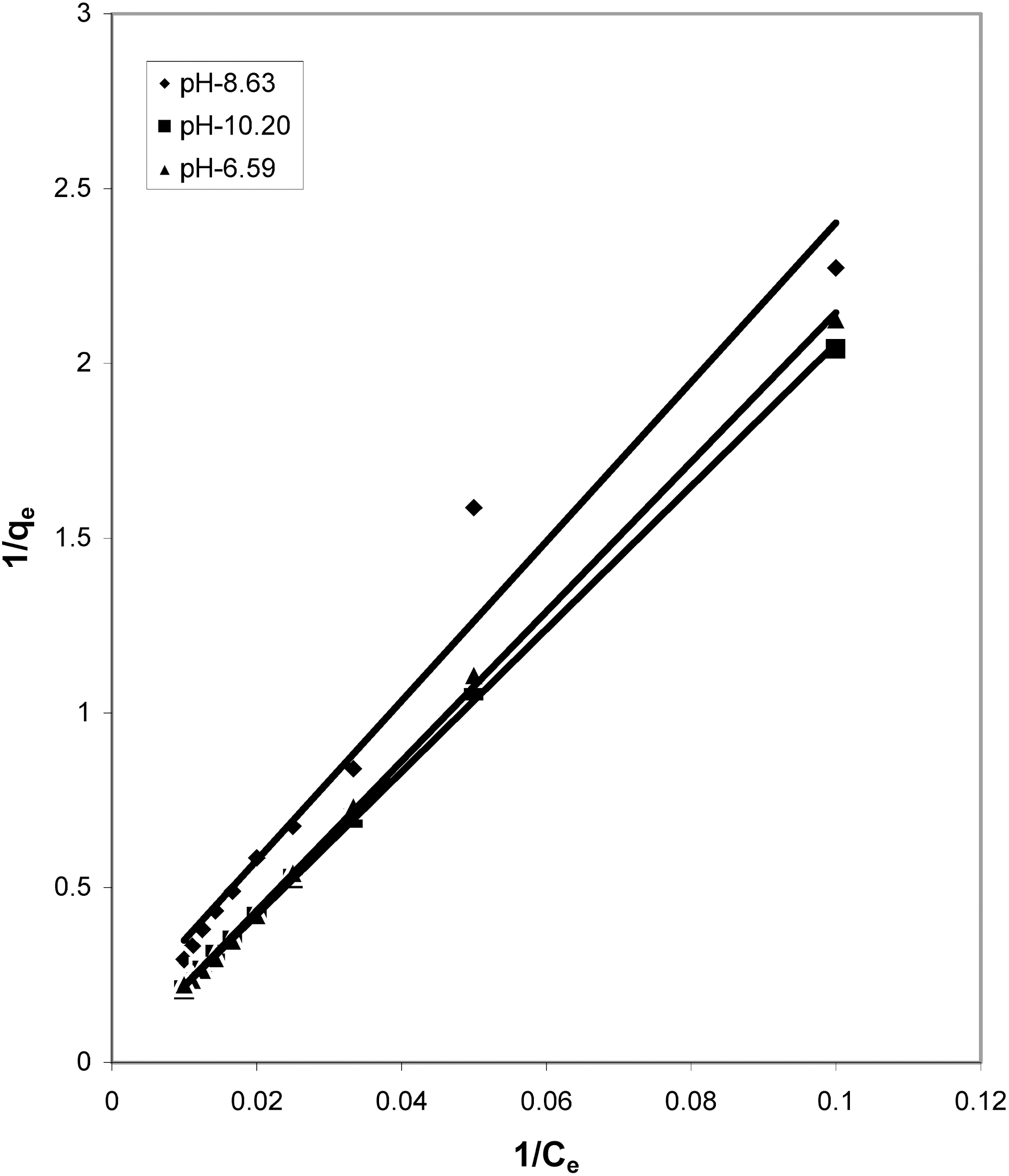
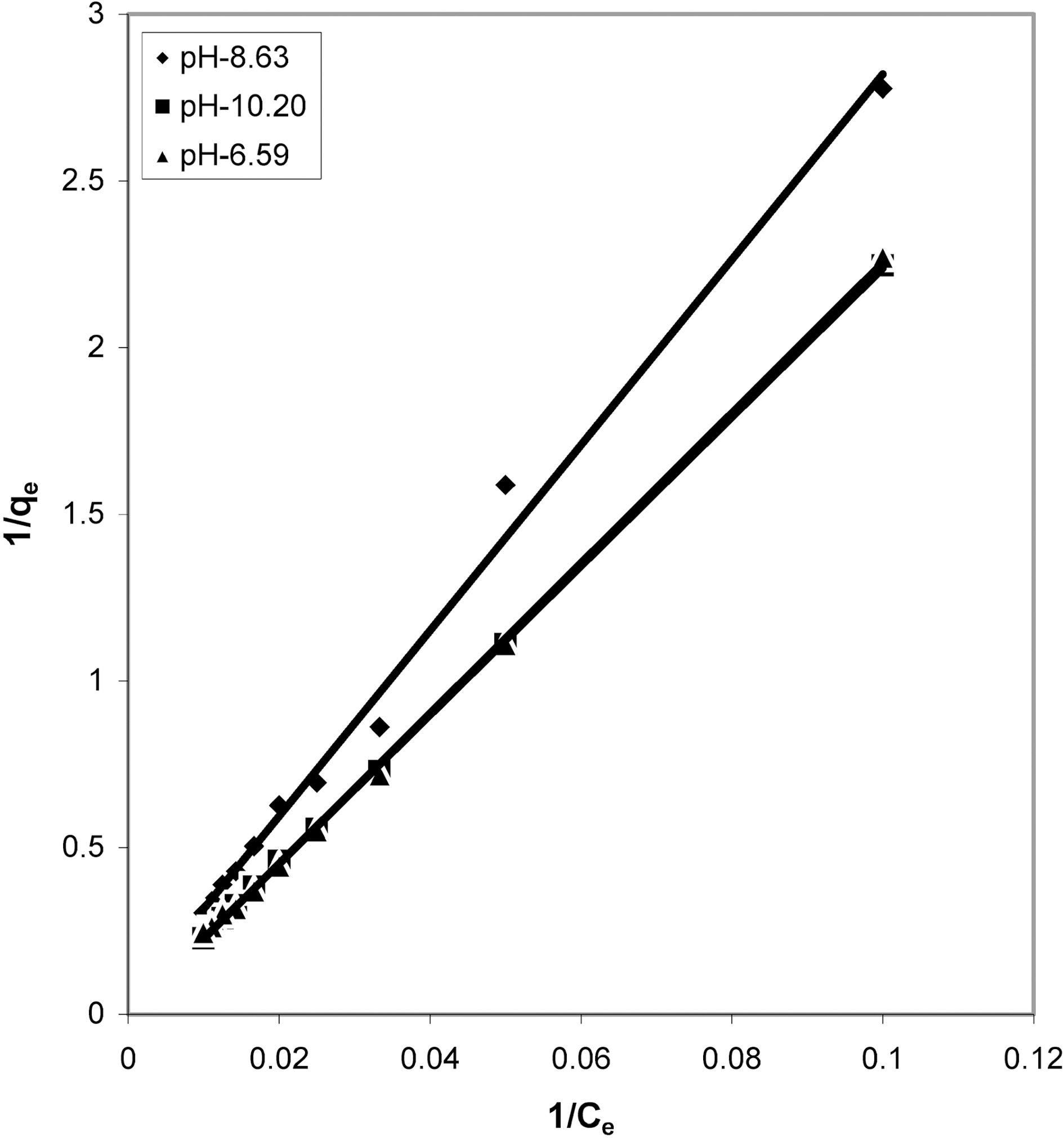
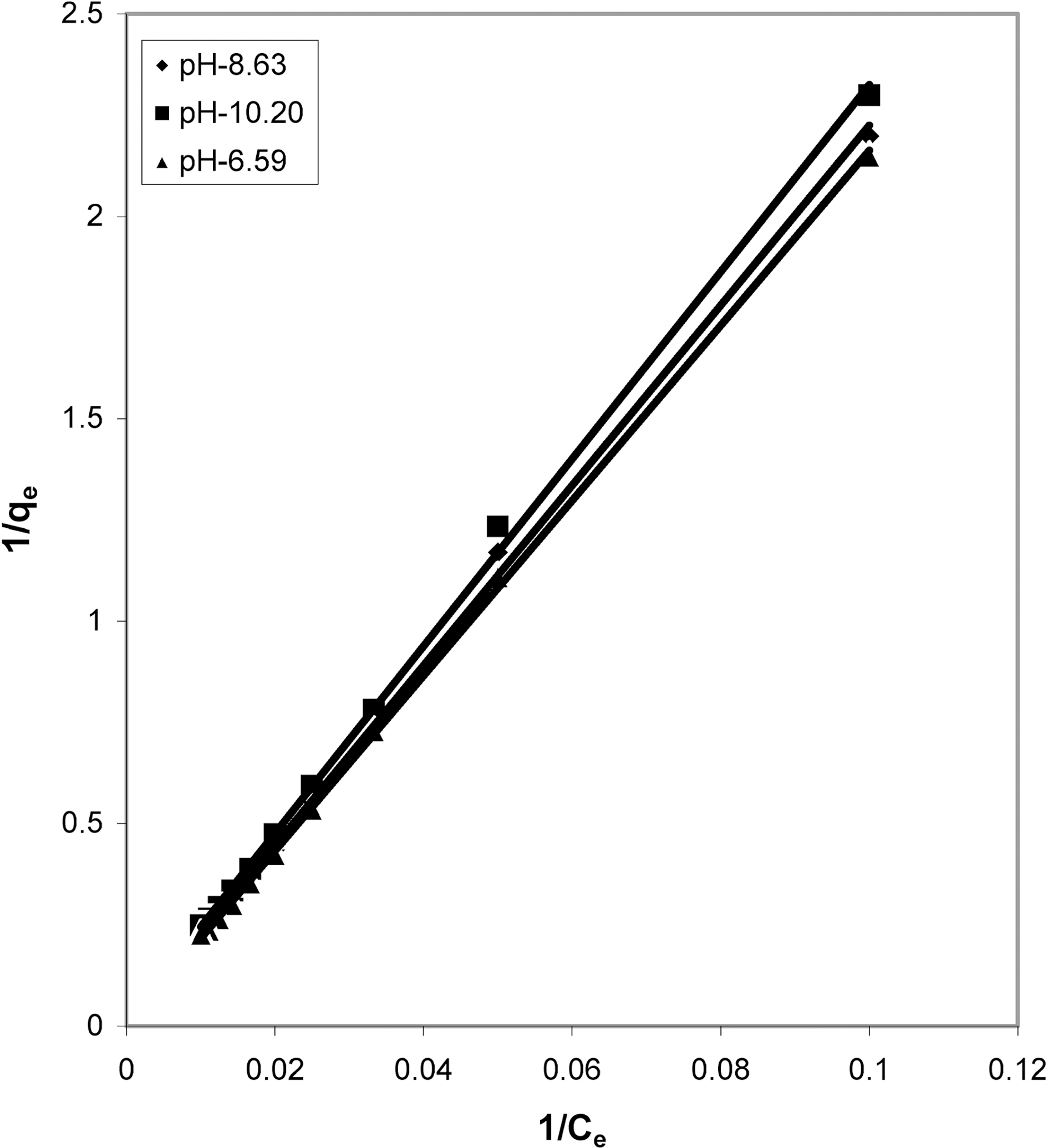
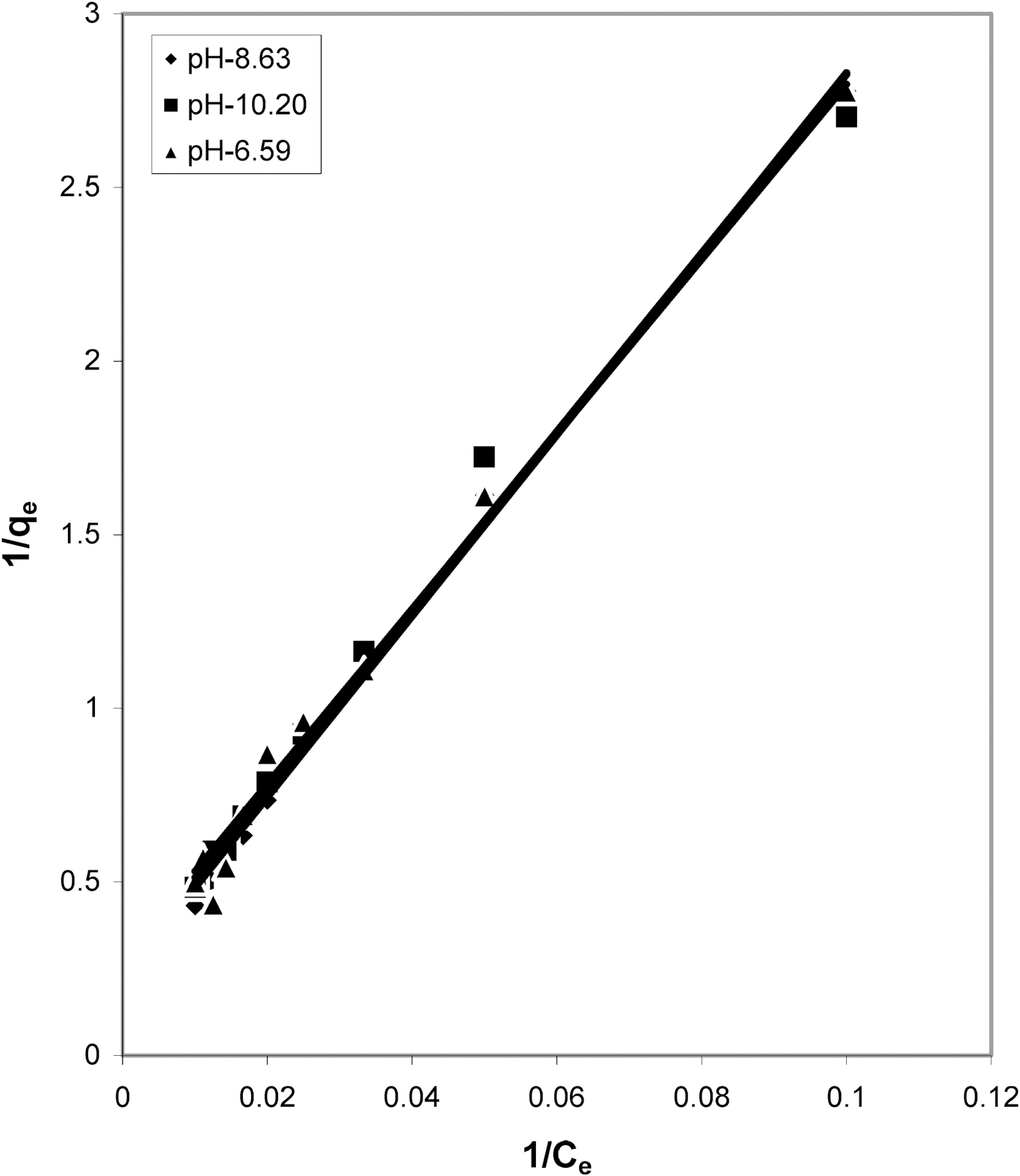
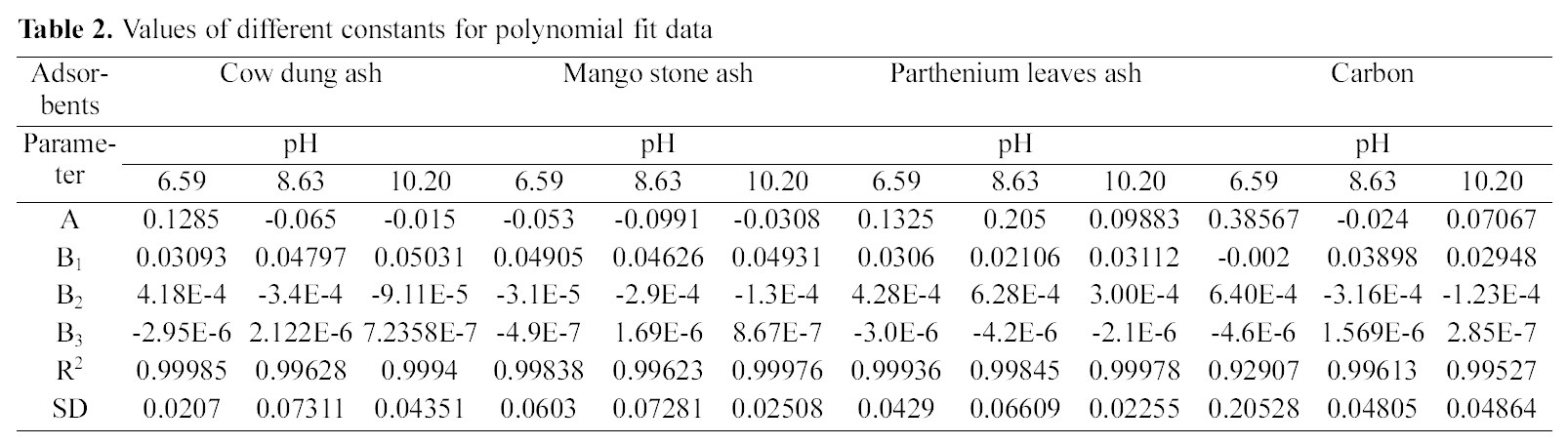
From the slope and intercept of linear plots between 1/qe
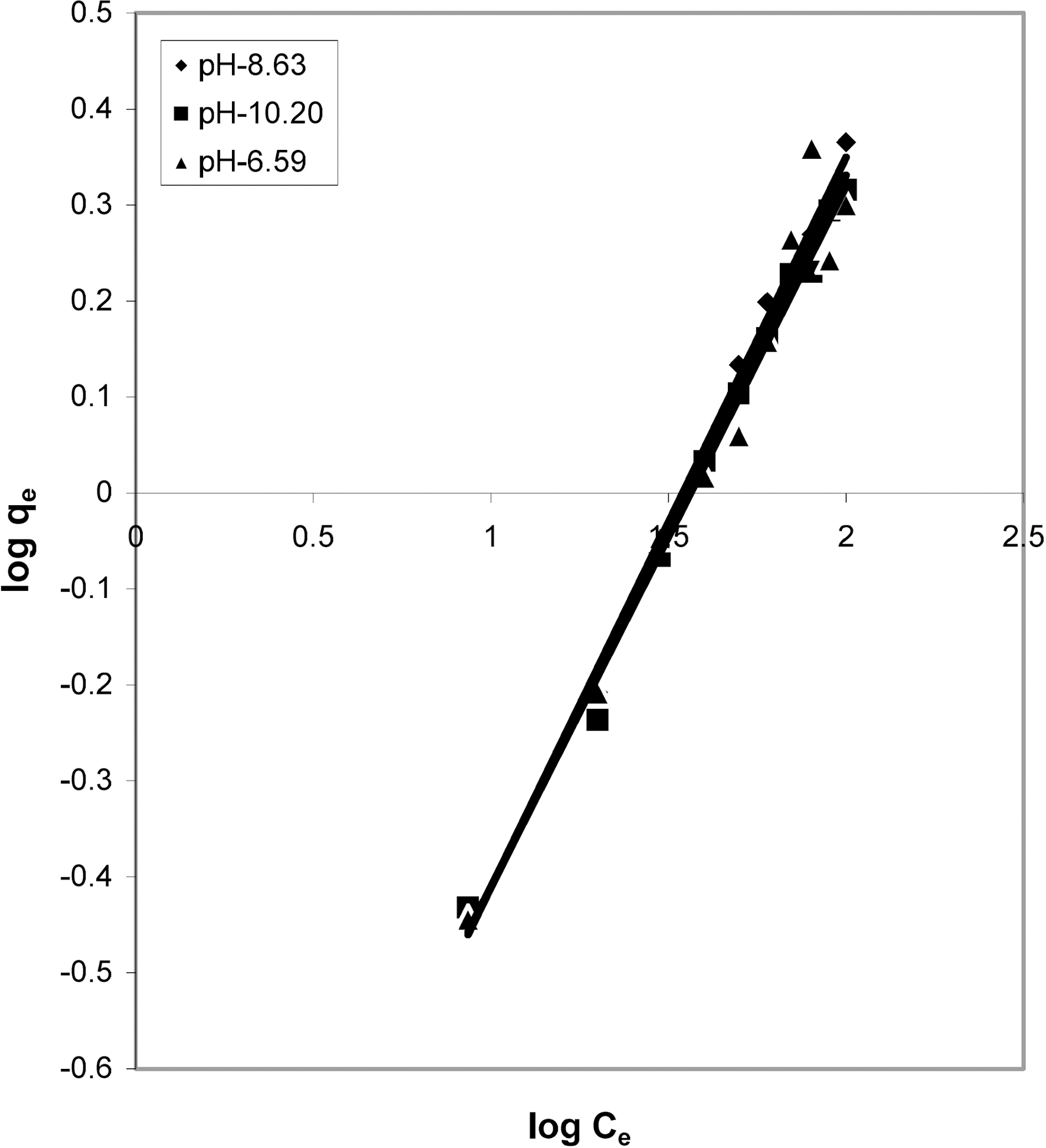
and 1/Ce as shown in Figs. 2, 5 and 8 and alsoFigs. 14 ~17 Langmuir constants “Q” and “b” were calculated. These values along with R and standard deviation are given in Table 3. Seeing the data it can be said that adsorption was favorable. Different values of Q are explained by varying
[Table 3.] Values of different constants for Langmuir isotherms at various pH
Values of different constants for Langmuir isotherms at various pH
affinity of dye towards the adsorbents.
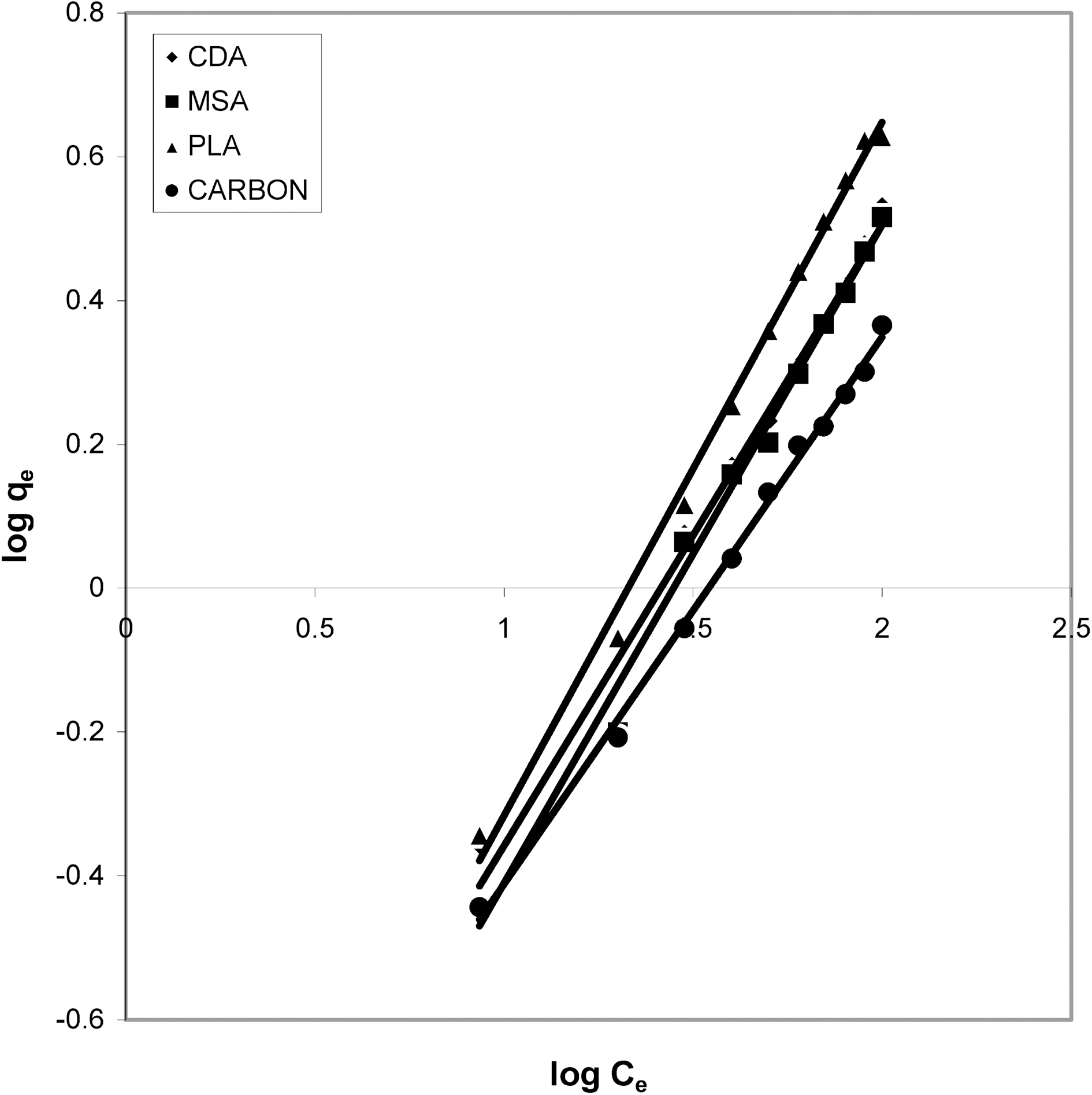
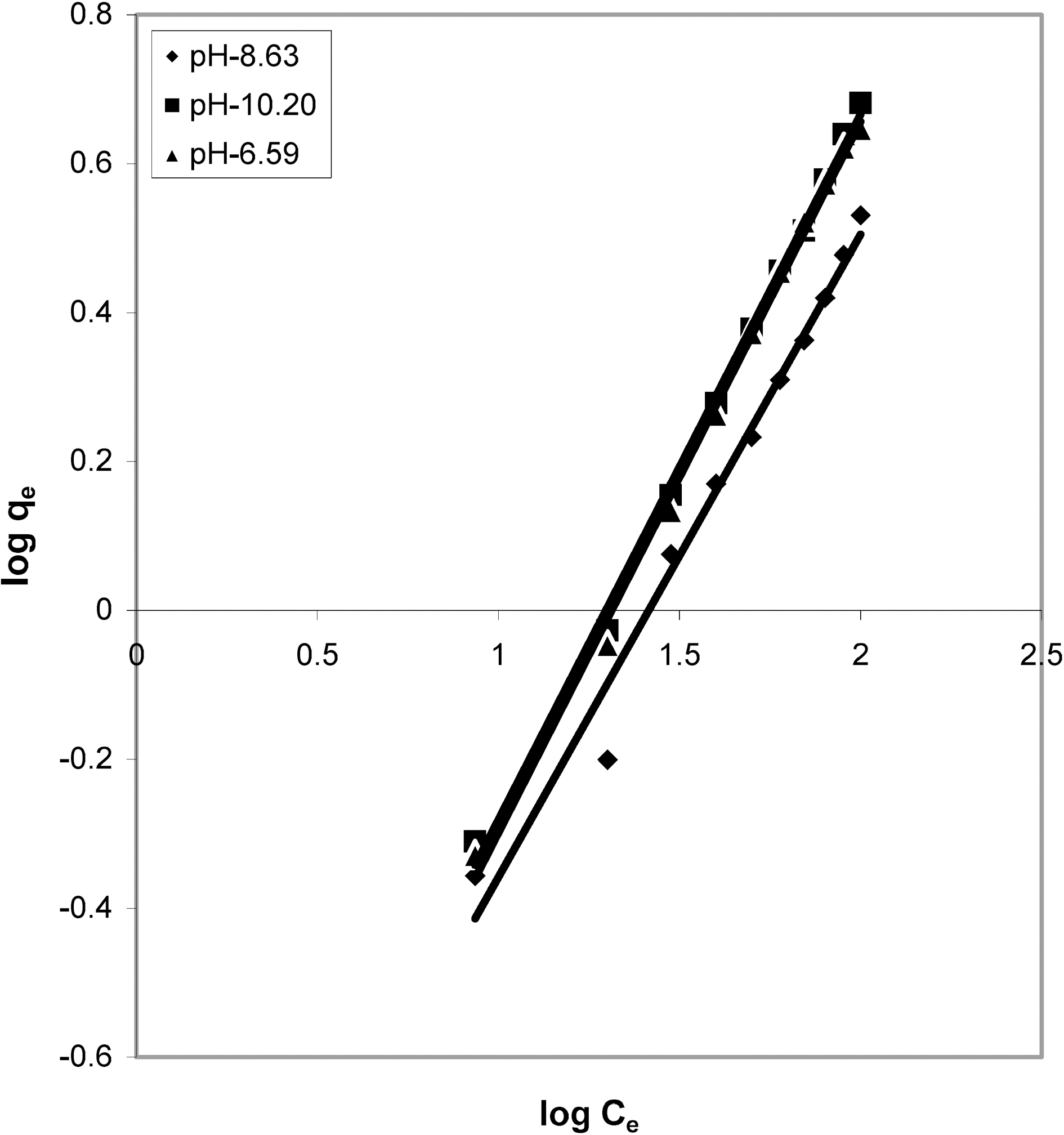
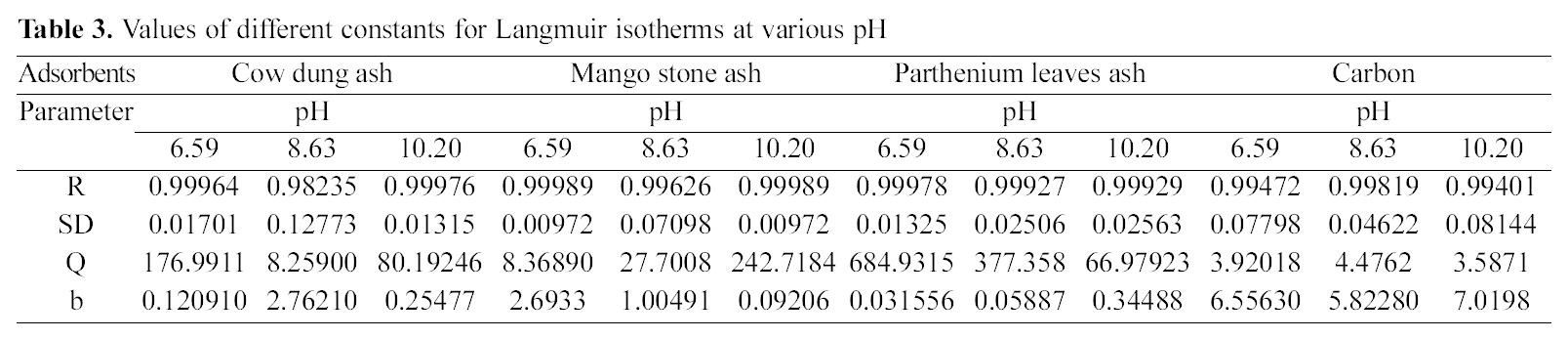
3.4. Freundlich isotherm at various pH
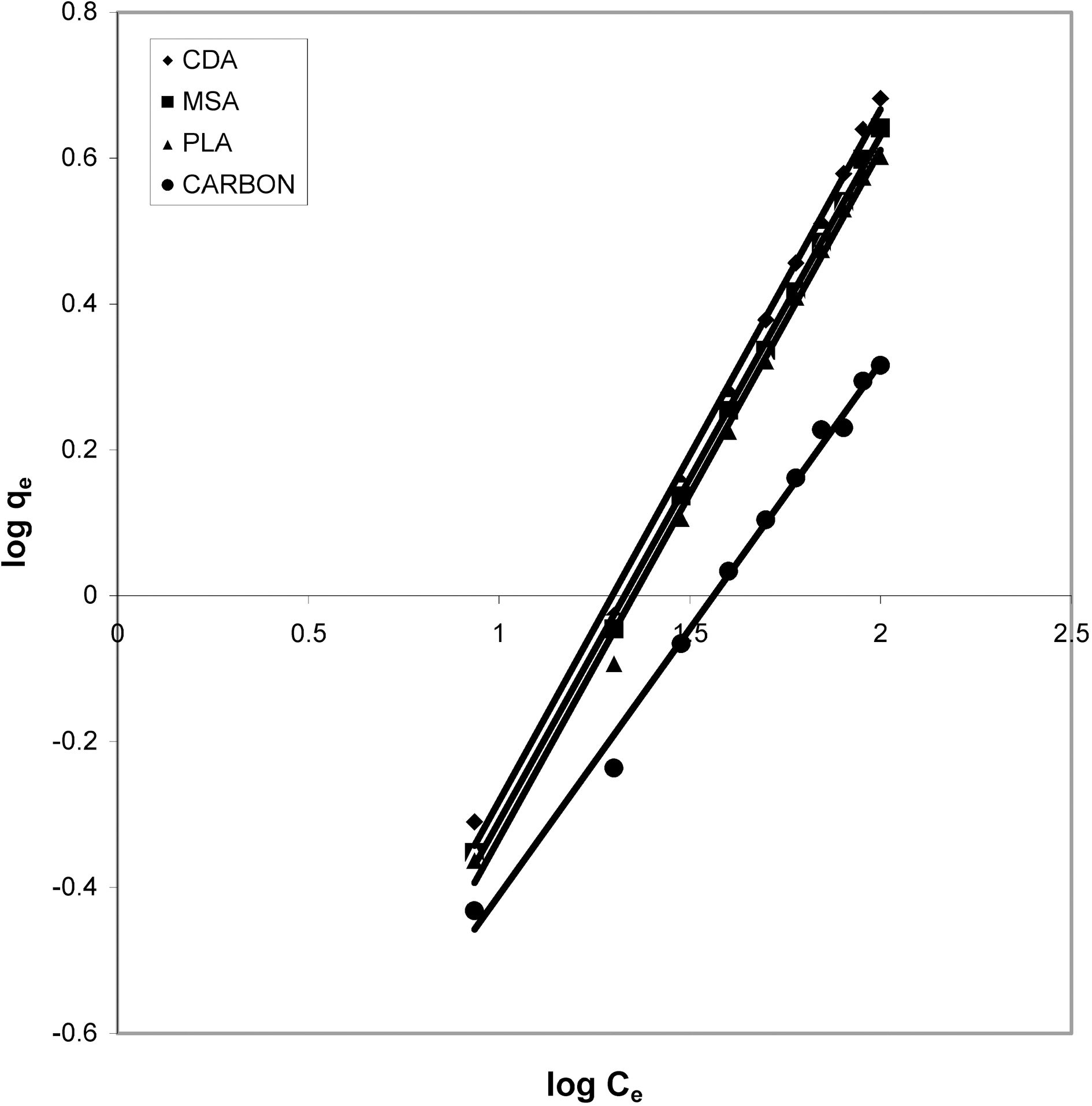
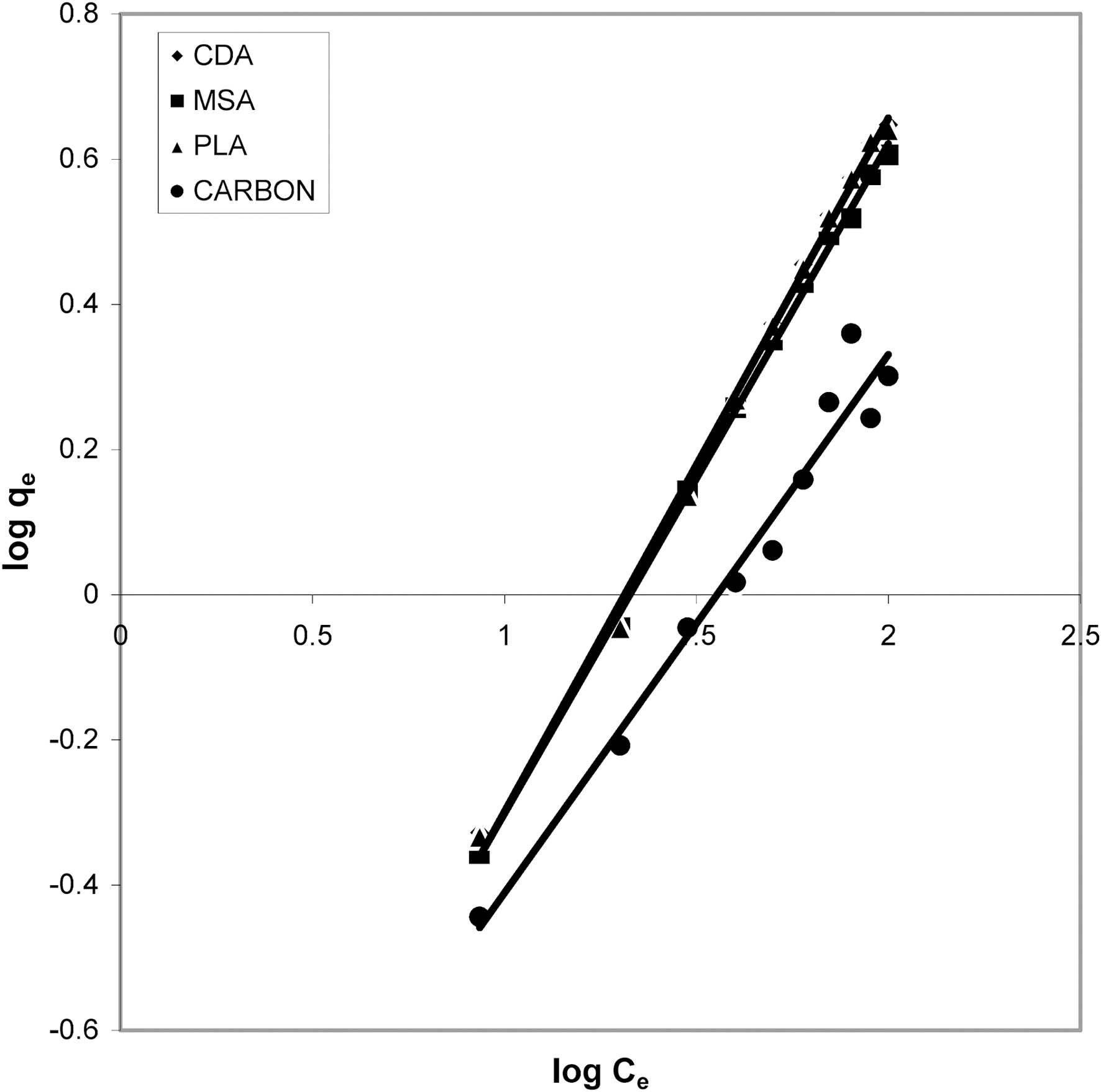
Figs. 3, 6 and 9 and also Figs. 18-21 show the linear plots of Freundlich isotherm log qe and log Ce. Freundlich constants KF and n calculated from the slope and intercept along with R and SD are given in Table 4.
Biosorption batch studies onto cow dung, mango stone ash, parthenium leaves ash and activated carbon have been performed in the present work employing Acid Green 20. Results indicate that there is a decrease in the percentage of dye removal of the dye per gram with increase in dye concentration. The pH of the aqueous solution played a significant role in affecting the adsorption capacity of the dye. The value of dimensionless separation factor R indicates that both adsorption isotherm models fitted satisfactorily and
[Table 4.] Values of different constants for Freundlich isotherms at various pH
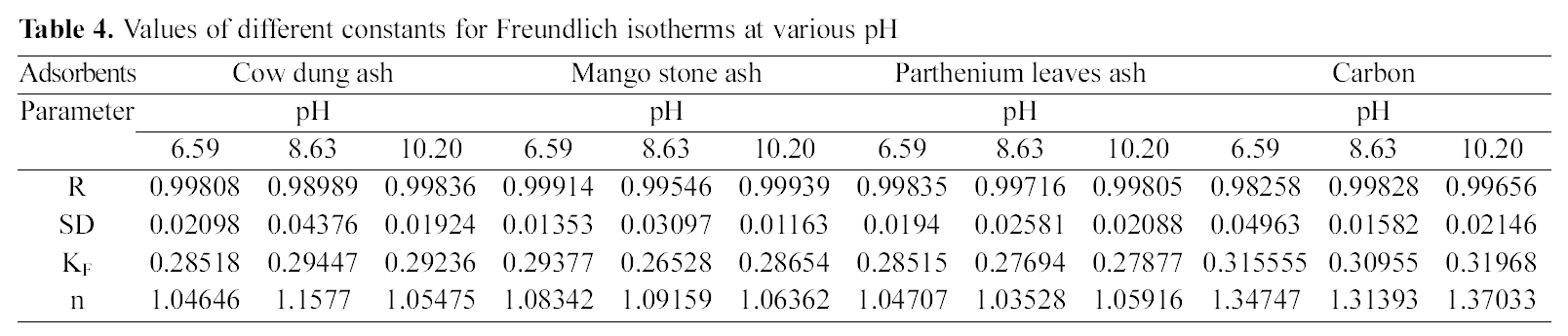
Values of different constants for Freundlich isotherms at various pH
suggested monolayer coverage on the outer surface of the adsorbent and the same also suggests that Langmuir gave a better fit. The different values of Q are explained by the varying degree of interaction between the adsorbate and the adsorbent. Consequently, safely can point to the use of these natural, ecofriendly materials due to their abundance and cheap biomass to minimize the burden of waste on the environment. This leads to their superiority as a potential sorbent in removal of some coloured dyes from wastewaters.