Carbon materials are the most actively used as anode materials for Li-ion secondary battery (LIB) due to their small surface change, stable structure, and favorable price. In order to apply LIB for electric vehicles (EV) and hybrid electric vehicles (HEV), they require not only high energy density but also high power density [1]. Therefore, the reversibility between charge and discharge of Li intercalation compounds used as the anode becomes of interest as an important factor for the battery mechanism [2,3].
Although the previous studies [4,5] of high capacity anode materials for LIB have focused on increasing amount of Li-ion intercalated, the studies [6-8] of high power anode materials emphasize the intercalation/deintercalation rate of Li-ion in the anode material structure. Graphite has a layer structure and the distance between the layers is about 3.35 A. When the expanded graphite (EG) having wider layer distance is applied to the anode materials for LIB, it is be assumed that the intercalation/deintercalation of Li-ion can be done with ease as compared with the case of graphite.
The objective of this study is to prepare a series of EG with different structures using ammonium peroxodisulfate as an oxidizing agent and high purity of sulfuric acid as an intercalate, and to apply the EGs as an anode material for high power LIB. We tried to find the suitable processing conditions of EG in order to apply the prepared EG as the anode material for high power LIB.
The flake type of natural graphite with well developed lamella structure (thickness: 10~15 ㎛) was used to make the EGs and the EGs were prepared by using ammonium peroxodisulfate ((NH4)2S2O8, Junsei Chem Co., Ltd, assay 98.0%) as an oxidizing agent and high purity of sulfuric acid(H2SO4, J. T. Baker. Co., Ltd, assay 96.6%) as intercalate. 20 g of natural graphite was added to a mixture of sulfuric acid(600 g) and calculated amount of ammonium peroxodisulfate in a double jacket reactor. The mixture was stirred at 98oC for 30 min and then dried in a oven at 80oC for 48 h after washing with distilled water until attaining neutralization.The dried samples were placed in a tube furnace reactor and heated to a desired temperature in the range of 800~1200oC maintaining 30~120 min at the temperature. The obtained EG samples were named as Ax-ty:Tz-tw, where Ax: the amount of ammonium peroxodisulfate (wt.%), ty: the acid treatment time (min), Tz: the heat treatment temperature(oC), and tw: the heat treatment time (min).
The anode active materials with particle sizes below 30 ㎛ were obtained by pulverizing the heat treated EG samples with a planetary mill and sieving at 400 mesh. The process variables such as the amount of oxidizing agent, the heat treatment temperature and heat treatment time were changed to find out the optimum manufacturing conditions of EG as the anode materials for LIB.
The crystallinity of various EG samples was analyzed by x-ray diffraction (XRD, Xpert-Pro, CuKa =1.54 A, PANalytical) and the microstructure of various EGs was observed by scanning electron microscope (SEM, 3500N, Hitachi Science System Ltd., Japan).
The electrodes were prepared by mixing 93 wt.% of the anode active materials and 7 wt.% of polyvinylidene fluoride(PVDF) binder dissolved in 1-methyl-2-pyrrolidinone (NMP)with a homogenizer at rotation speeds of 4000~5000 rpm to form slurry. The mixed slurry was then coated on the copper foil by the Doctor Blade method and dried at 100oC in a vacuum oven for 24 h before being compressed by a roll press at 80oC. The prepared anodes with 2.5×2.5 cm size were kept in a glove box filled with argon where the contents of moisture and oxygen were controlled below 1 ppm. The prepared electrode was used as a working electrode and lithium foil adhered to copper mesh with 3.0×3.0 cm size was used as a counter electrode. 1 M LiPF6 salt dissolved in a mixture of ethylenecarbonate (EC),ethylmethylcarbonate (EMC), and dimethylcarbonate (DMC) at 1:1:1 volume ratio was used as the electrolyte. The half cells were assembled in a glove box with argon atmosphere using polypropylene (PP® ; Celgard 2400 microporous membrane)separator to separate the cathode and anode.
The electrochemical properties of half cells were estimated by using a WBCS-3000 battery charge/discharge cycler(WonA Tech Co., Ltd.) at a constant temperature of 25oC.The charge characteristics were measured by applying the constant current (CC) method at 0.2 C for the first charge until reaching to 0.005 V and then changing to the constant voltage (CV) method for the successive charge by reducing the current until 2.5% of the initial current was attained. As for discharge, the CC method was applied at 0.2 C until reaching to 2 V. The current rates in the CC method were increased to 1 and 10 C for the high rate charge-discharge characteristics. Here 1 C meant a rate of current where the full charge or discharge for the given anode sample would take 1 h assuming 270 mAh/g of capacity.
Fig. 1 shows the SEM image of various EGs prepared using different amounts of oxidizing agent. The distance between the layers was increased with increasing the amount of oxidizing agent. As the result, the specific surface area
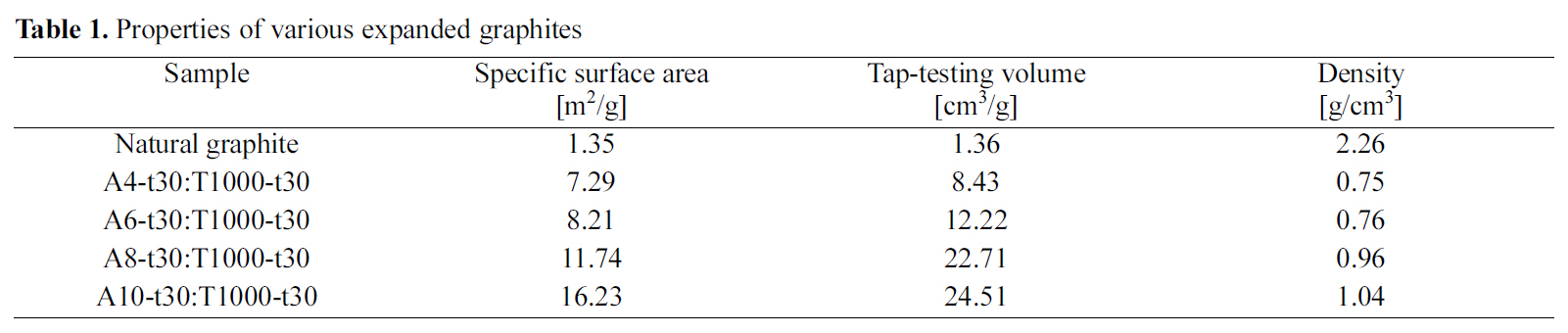
[Table 1.] Properties of various expanded graphites
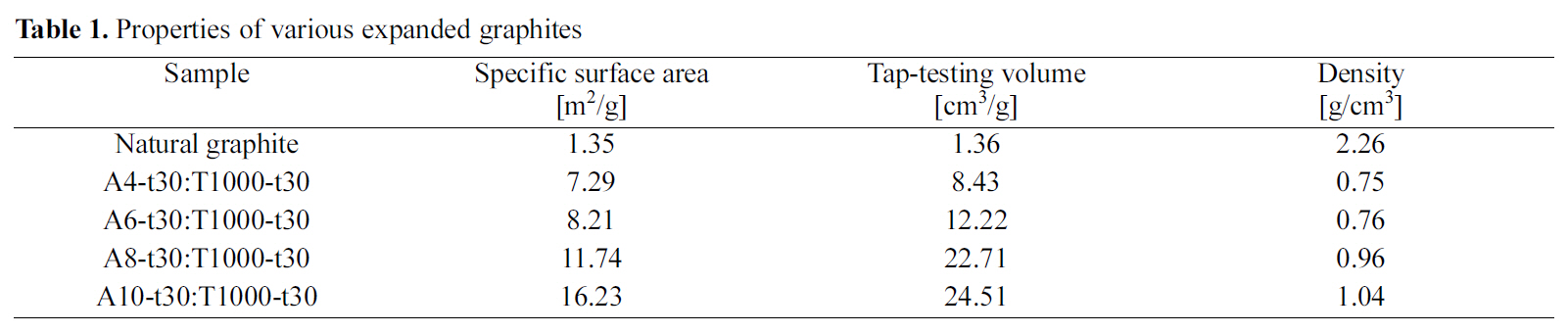
Properties of various expanded graphites
and tap-testing volume increased significantly with increasing the amount of oxidizing agent as shown in Table 1.
According to the previous works [9,10], the S2O8 2- species formed by Eq. (1) and (2) causes weakening of van der Waals force between the graphite layers. As a result, the intercaltion of graphite is occurred by the introduction of HSO4- into the gap of graphite layers. Therefore, the chemical equation for the intercaltion of graphite by sulfuric acid can be expressed as Eq. (3).
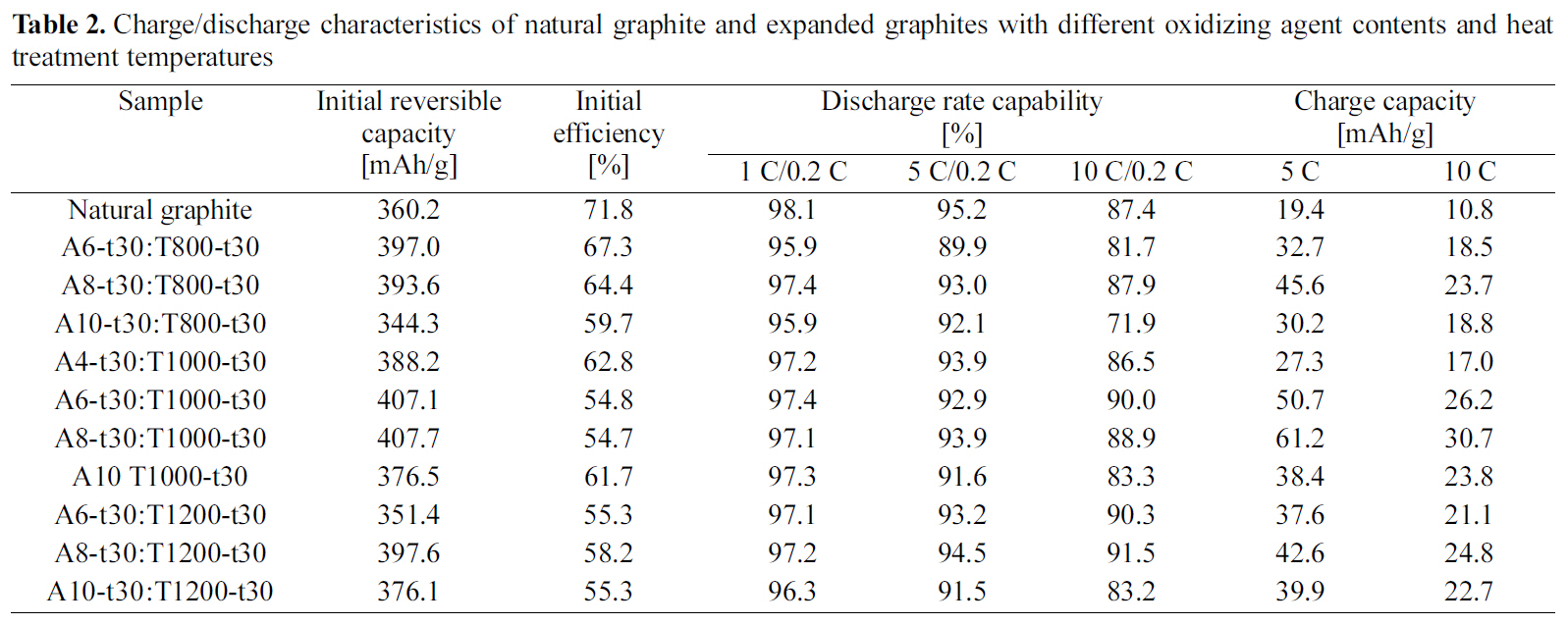
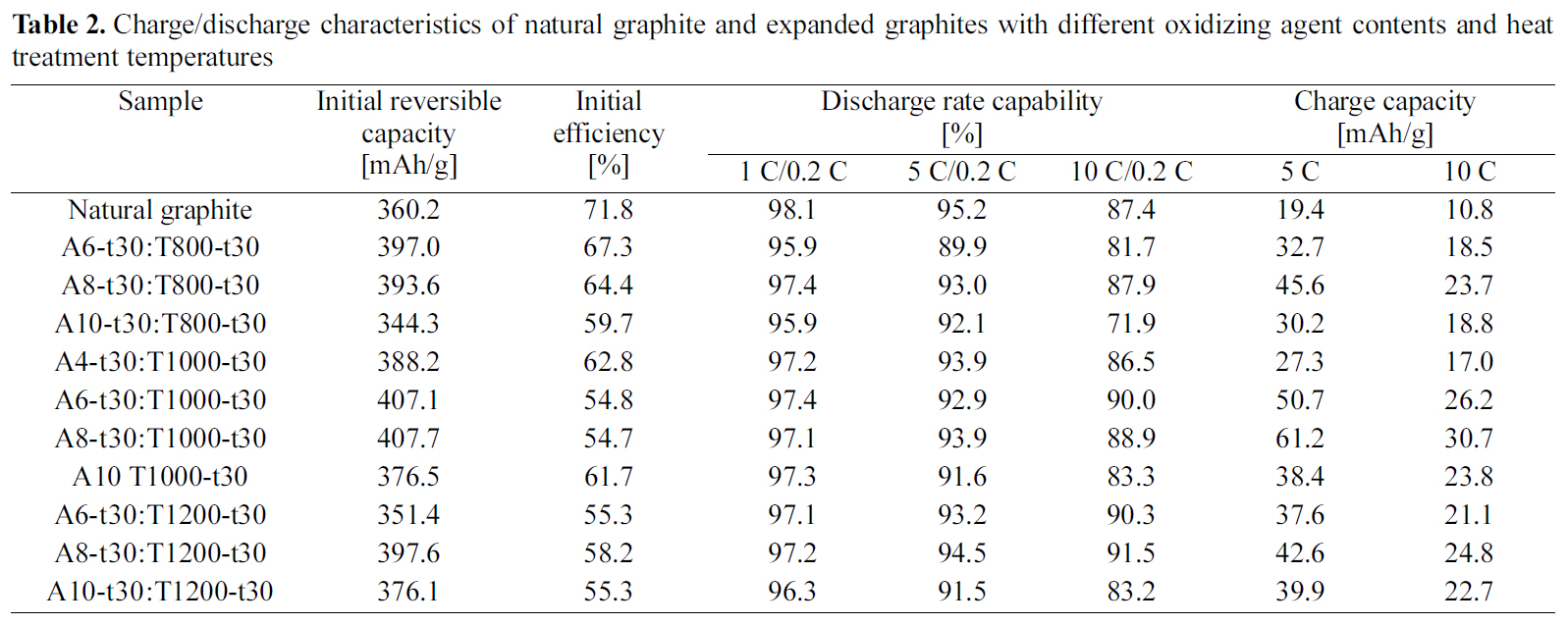
Charge/discharge characteristics of natural graphite and expanded graphites with different oxidizing agent contents and heat treatment temperatures
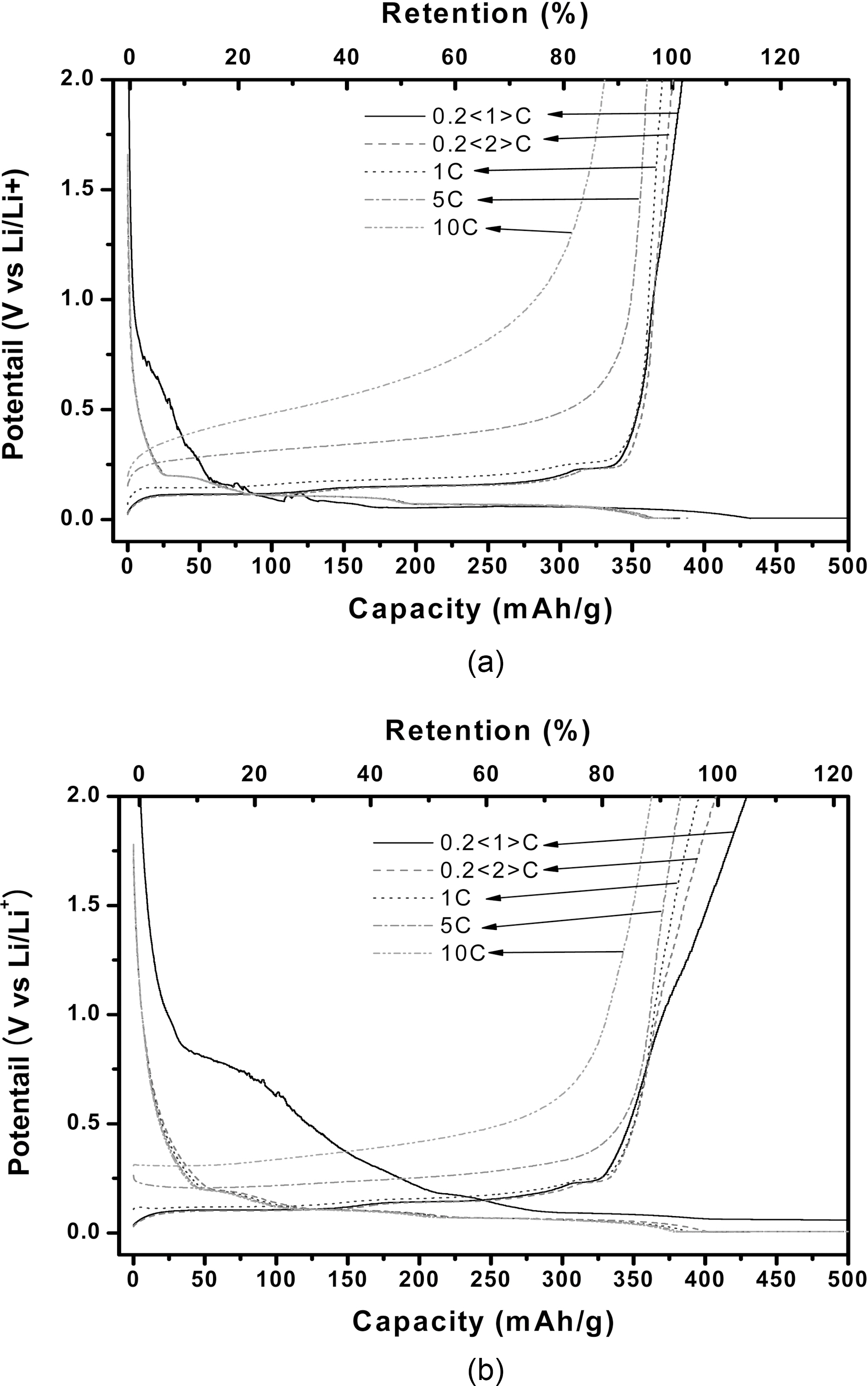
The charge-discharge curves of natural graphite and EG(A8-t30:T1000-t30) as a function of C-rate are presented in Fig. 2. Although the expanded graphite showed the characteristics of potential plateau similar to the natural graphite, it provided the higher reversible capacity with the more declined curve to the right in the range of 0.05~2 V as compared with the natural graphite. Table 2 represents the charge-discharge characteristics of anodes for natural graphite and EGs with different oxidizing agent contents and heat treatment temperatures. With increasing the amount of oxidizing agent, the charge/discharge characteristics of EG increased up to 8 wt.% and then it decreased with the further increase. The analysis of SEM and XRD revealed that the layer structure of graphite was expanded uniformly allowing the easier intercalation/deintercalation of Li-ion up to 8 wt.%of oxidizing agent. However, the EG with the oxidizing agent content over 10 wt.% showed that the distance of graphite layer excessively widened differentiating from those with the lower oxidizing agent contents.
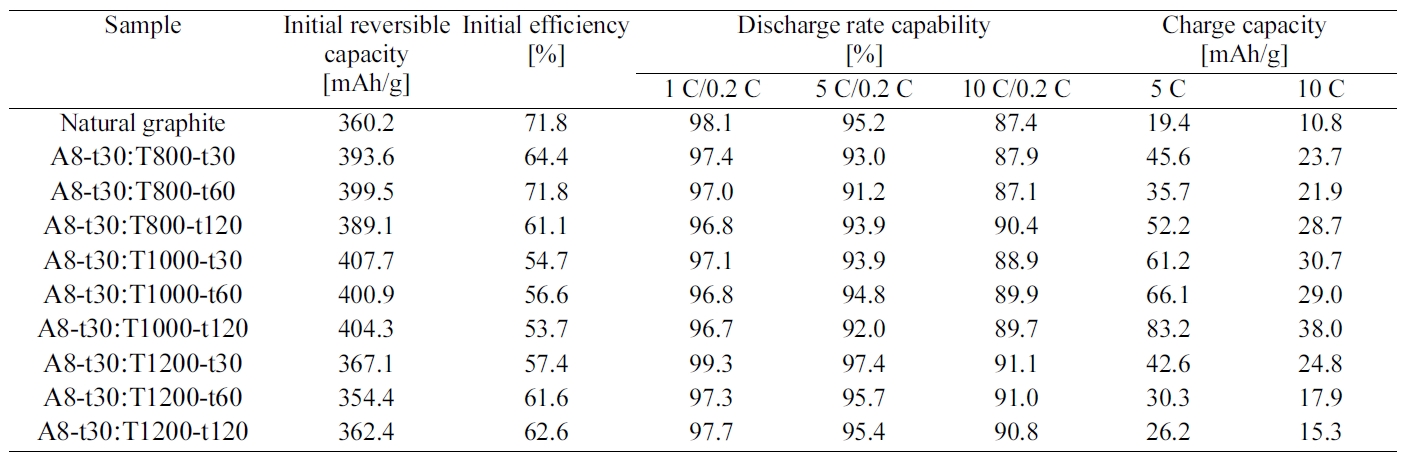
When the intercalated samples were heat treated in the range of 800~1200oC under nitrogen atmosphere, the charge/discharge characteristics was increased until 1000oC, but it was largely decreased with the heat treatment at 1200oC as shown Table 2 and
After checking the electrochemical analysis of various EG anode samples prepared at the different conditions, the optimal conditions were determined as the oxidizing agent of
8 wt.% with 30 min's acid treatment and further heat treatment at 1000oC for 120 min. The sample (A8-t30:T1000-t120) showed the improved charge/discharge characteristics such as 404.3 mAh/g of initial reversible capacity, 90.8% of discharge rate capability at 10 C-rate, and 83.2 mAh/g of charge capacity at 5 C-rate. However, this EG anode still had potential plateaus, which is not favorable for high power Li-ion secondary battery, and showed the decreased initial efficiency of 53.7% as compared with that of natural graphite (71.8 %).
In order to apply as the anode material for high power LIB, a series of EG with different structures were produced by changing the processing conditions such as the amount of oxidizing agent, and the heat treatment temperature and time.By using different amount of ammonium peroxodisulfate as the oxidizing agent and changing the heat treatment temperature, the behavior of intercalation compound(H2SO4) and the chemical interaction in the surface, and the distance of graphite layers were influenced with the complex changes in the EG structure. With increasing the oxidizing agent, the interlayer distance between graphite layers was expanded and the more disordered structure was developed,showing the volume expansion and the increased specific surface area. The optimal conditions were determined as 8 wt.% of the oxidizing agent with 30 min's acid treatment and further heat treatment at 1000oC for 120 min. The optimized anode sample showed the improved charge/discharge characteristics such as 404.3 mAh/g of initial reversible capacity, 90.8% of discharge rate capability at 10 C-rate, and 83.2 mAh/g of charge capacity at 5 C-rate, as compared with those for the natural graphite anode, showing the possibility of EG anode material for high power LIB.