Radiation-induced graft polymerization (RIGP) is a useful method for the introduction of various functional groups into different polymeric materials using specially selected vinyl monomers. There are several reports about RIGP of polar monomers onto polymer substrates with hydrophobic properties in order to obtain hydrophilic properties for versatile application [1-5]. In previous papers [6,7], RIGP was also performed using various vinyl monomers in the presence of multi-walled carbon nanotubes (MWNTs) in an aqueous solution at room temperature to introduce functional group onto MWNTs surface for electrochemical biosensor supports.
In recent years, direct electrochemistry of biologically important enzymes have been studied with ion liquids (ILs) in both theoretical and practical applications because ILs are considered to be suitable media for supporting a biocatalytic process with high polarity, non-coordination power, high selectivity, fast rate and great enzyme stability [8,9]. Enzymes are usually active and protein refolding is improved in ILs [10]. Zeng and coworkers fabricated a modified GC electrode by GOx into a nano Au particle-ion liquid-
On the other hand, caffeine is an active alkaloid present in the coffee and thus determined of caffeine is required in food laboratories in order to inform the consumers about characteristics of coffee samples. There are several methods proposed in the literature for the determination of caffeine in foods, based on ultraviolet-visible spectroscopy [14], mass spectrometry [15,16], gas chromatography [17,18], and high performance liquid chromatography (HPLC) [19,20]. Electrochemical methods can be characterized the caffeine in food by simplicity, high sensitivity, good stability, low-cost instrumentation and on-site monitoring. However, a little has been reported about electrochemical determination of the caffeine in food until now.
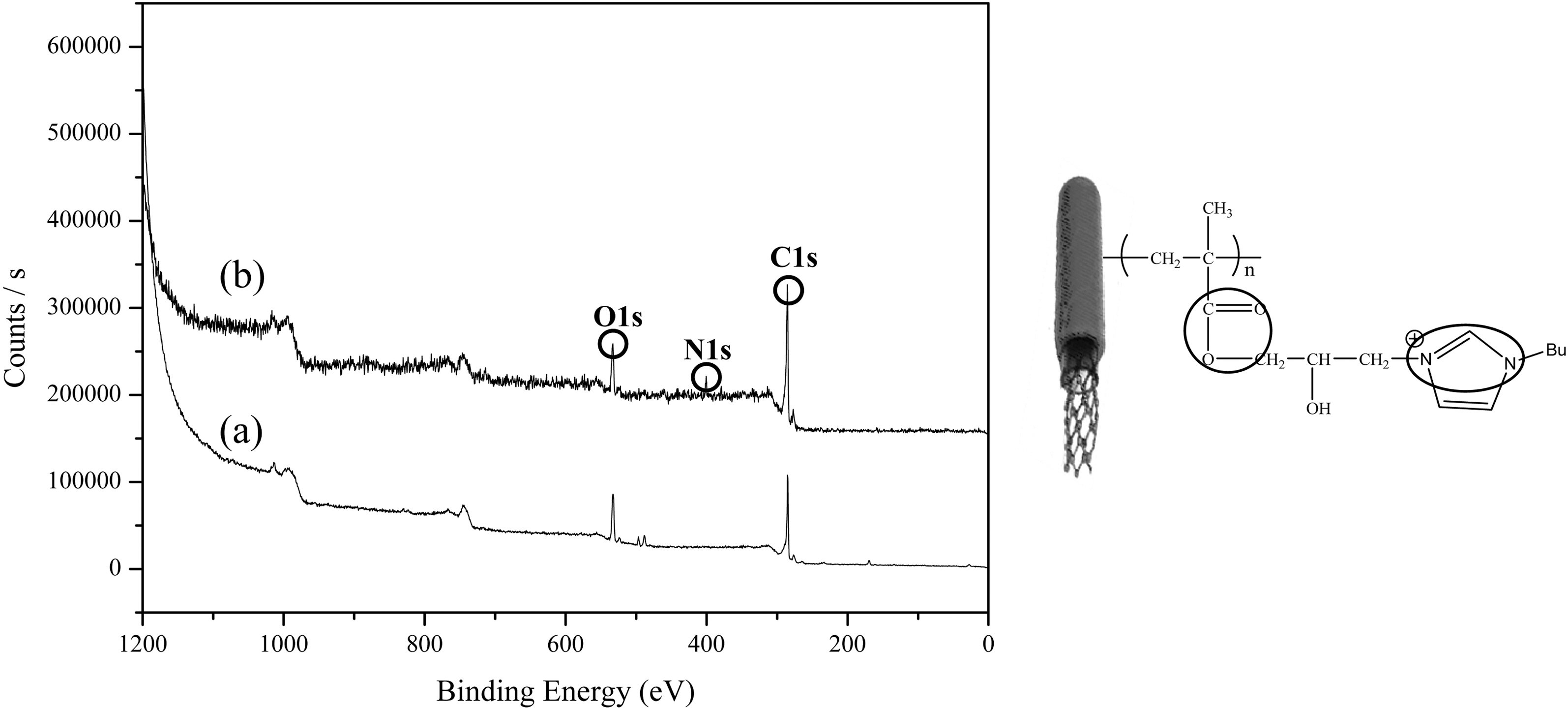
In this study, we synthesized two-type vinyl monomers with ionic properties, 3-(butyl imidazol)-2-(hydroxyl)propyl methyl methacrylate and 1-[(4-ethenylphenyl)methyl]-3-buthyl-imidazolium chloride by ordinary chemical reaction in acetonitrile. Next, the ionic properties were introduced onto MWNTs surface by RIGP of vinyl monomers in aqueous solution at room temperature. Finally, the
Scheme 1. Synthesis procedure of ion-modified MWNTs by radiation-induced graft polymerization.
Scheme 2. Synthesis procedure of IL-modified MWNTs by radiation-induced graft polymerization.
sensing efficiency for phenol in a phosphate buffer solution. Total phenolic compounds, mainly caffeine, for four-type commercial coffee were also examined using the prepared
Glycidyl methacrylate (GMA), buthyl imidazole, styrene methylchloride, and chitosan were of analytical reagent grade (Aldrich?Sigma) and used without further purification. An ITO electrode as a working electrode (working area 0.7×1.1 cm2, 10 W resistance) was purchased from Dasom RMS Co. (Korea). MWNTs (CM-95) were supplied by Hanwha Nanotech Co., Ltd (Korea). Solutions for the experiments were prepared with water purified in a Milli-Q puls water purification system (Millipore Co. Ltd., the final resistance of water was 18.2 MΩcm-1) and degassed prior to each measurement. Other chemicals were of reagent grade.
2.2. Preparation of ionic property-modified MWNTs by radiation-induced graft polymerization
Scheme 1 and 2 shows the preparation procedure of ionic property-modified MWNTs by radiation induced graft polymerization of the ionic property-modified vinyl monomers. We firstly synthesized two-type ionic property-modified vinyl monomers as follows: 3-(butyl imidazol)-2-(hydroxyl) propyl methyl methacrylate was synthesized by reaction of GMA (5.34ml, 0.04mol) and butyl imidazole (5.45ml, 0.04mol) in acetonitrile (50ml) at 60℃ for 16 h as similar method of the earlier literature [21]. The obtained product was placed into Et2O, and then crystallized in a freezer for several hours. The solid state product was obtained decantation, and dried in vacuum oven at room temperature. Yield=42.3%, LC-Mass =267.3.1-[(4-Ethenylphenyl)methyl]-3-buthyl-imidazolium chloride was also synthesized according to the reported literature [22]. In detail, a butyl imidazole (5.45 ml, 0.04 mol) was dissolved in acetonitrile (30 ml) in a 100 mL round bottom flask equipped with a stir bar. 4-vinylbenzyl chloride (6.94 ml, 0.04 mol) was then added, and the reaction was heated 50℃ while stirring overnight. The reaction was stopped after this time, and the reaction mixture was inserted into Et2O, and then placed in a freezer for several hours. The solid state product was obtained decantation, and dried in vacuum oven at room temperature. Yield =66.4%, LC-Mass= 301.2.
In order to use MWNTs as supports, MWNTs were purified to remove the catalyst and non-crystallized carbon impurities. MWNTs were treated with phosphoric acid. The purified MWNTs were used as the supporting materials for grafting of various vinyl monomers.
The ionic property-modified MWNTs were prepared as following method. The MWNTs (2.0 g) and the prepared vinyl monomers with ionic property (2.0 g) were mixed in aqueous solution (20 mL). Nitrogen gas was bubbled through the solution for 30 minutes to remove oxygen gas, and the solution was irradiated by γ-ray from a Co-60 source under atmospheric pressure and ambient temperature. A total irradiation dose of 30 kGy (dose rate=1.0× 104 Gy/h) was used. The obtained samples was separated by centrifuge with 2000 rpm, and then dried in vacuum oven at 50℃ for 18 h after then separated.
2.3. Fabrication of the tyrosinase-immobilized biosensor
Tyrosinase-immobilized biosensor based on ionic property-modified MWNTs were prepared as follow: the mixed solution containing chitosan (3.2 mg) as a binder,tyrosinase (25,000 unit, 0.3 mg), and ionic property-modified MWNTs (3.2 mg) as the supports in an acetic acid solution (0.01 mL) was prepared, and then the mixed solution (9.0 μL) was coated on the surface of a pre-cleaned ITO electrode by hand-cast method. The prepared MWNTs ion liquid electrode was keep at 4℃ until being used.
Cyclic voltammetric experiments were performed with a Potentiostat/Gavanostat model 283 (Ametek PAR, U.S.A.). All experiments were carried out with a conventional threeelectrode system. The working electrode was an ITO electrode coated with the ionic property-modified MWNTs, counter electrode was the platinum wire, and reference electrode was an Ag/AgCl (sat’d KCl). The surface morphology of the samples was determined by Scanning Electron Microscopy (SEM, S-3000N, Hitachi Science System Ltd., Japan) and HR-TEM (JEOL, JEM-2010, USA). The Xray photoelectron spectra of the samples were obtained using MultiLab ESCA2000 (Thermo Fisher Scientific). The thermal gravimetric analysis (TGA) was conducted on a Scinco TGA S-1000 (Seoul, Korea) under N2 flow from 25℃ to 700℃ at a heating rate of 20℃/min. The molecular weight of the samples was determined by liquid chromatography-mass spectrometer (LC/MS, 1100 + G1958, Agilent Technologies, USA).
3.1. Preparation of ionic property-modified MWNTs
In a previous paper [23], the various vinyl monomers such as acrylic acid, methacrylic acid, glycidyl methacrylate, maleic anhydride, and vinylphenyl boronic acid was grafted onto MWNTs by radiation-induced graft polymerization at room temperature in aqueous solution. We selected vinyl monomers because vinyl monomers are possessed hydrophobic properties of the vinyl group and hydrophilic properties of functional group part on vinyl monomers. The vinyl group of vinyl monomers is faced to the MWNTs surface because of its hydrophobic-hydrophobic interaction, while the functional group of vinyl monomers is faced in aqueous solution because of its hydrophilic properties. At that time, the radical polymerization of vinyl monomers is performed on the surface of MWNTs during γ-irradiation. As result, we can successfully introduce the various functional groups onto MWNTs surface as like tubular-type morphology. We can easily prepare the ionic property-modified MWNTs by RIGP according to the similar method as mentioned above. The ionic property-modified MWNTs can apply as supports of the immobilization enzyme because of strong cationic property. So we introduce the ionic propertymodified vinyl monomers onto MWNTs surface by RIGP in aqueous solution at room temperature by one-step reaction.
Fig. 1 shows the XPS survey scan spectra of pure MWNTs (a) and ion-modified MWNTs prepared by RIGP. There were significant changes after grafting of 3-(butyl imidazol)-2-(hydroxyl)propyl methyl methacrylate onto the pure MWNTs in the XPS data. The characteristic N 1s peak at 399 eV appeared after grafting of 3-(butyl imidazol)-2-(hydroxyl)propyl methyl methacrylate onto the pure MWNTs. This illustrates that the cationic property-modified MWNTs was successfully prepared by the RIGP method.
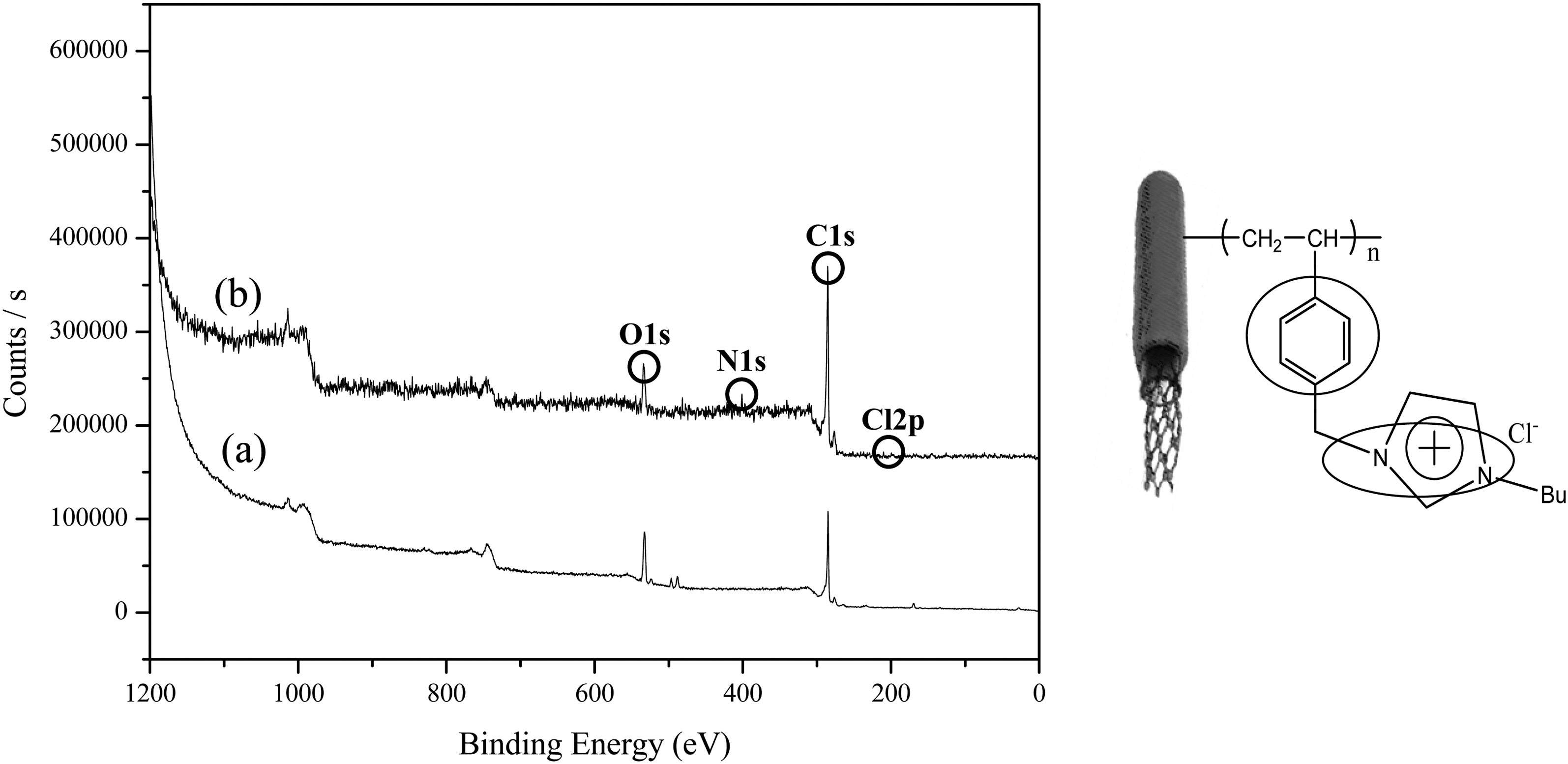
Fig. 2 exhibited the XPS curves of the purified MWNTs (a) and ionic liquid (IL)-modified MWNTs prepared by RIGP. There were prominently change after radiation grafting of 1-[(4-ethenylphenyl)methyl]-3-buthyl-imidazolium chloride onto pure MWNTs in the XPS data. The
characteristic N 1s peak at 399 eV and Cl 2p at 200 eV exhibited after grafting of vinyl monomer with IL property by RIGP onto pure MWNTs at room temperature by onestep reaction. As result, the vinyl monomers with IL property were successfully prepared via RIGP methodology, by “one-pot radical polymerization”.
Fig. 3 shows the TEM images of the purified MWNTs (a), and ion-modified MWNTs (b) and IL-modified MWNTs prepared by RIGP (c). These images clearly show a fine coating on the surface of MWNTs, thereby the diameter of MWNTs
increased. In TEM image of Fig. 3, the diameter of the purified MWNTs, Fig. 1-(a) and the coated MWNTs, Fig. 1-(b and c) was at 11±0.05 nm, 20±0.05 nm, and 17±0.05 nm,
respectively. The ionic property (ion and IL)-modified MWNTs are thicker than the purified MWNTs. As result, the ionic property-modified MWNTs were successfully prepared by one-step radiation graft polymerization. These ionicmodified MWNTs can be efficiency immobilized biomolecules such as enzyme, microbial, protein and etc. because of having plus charge.
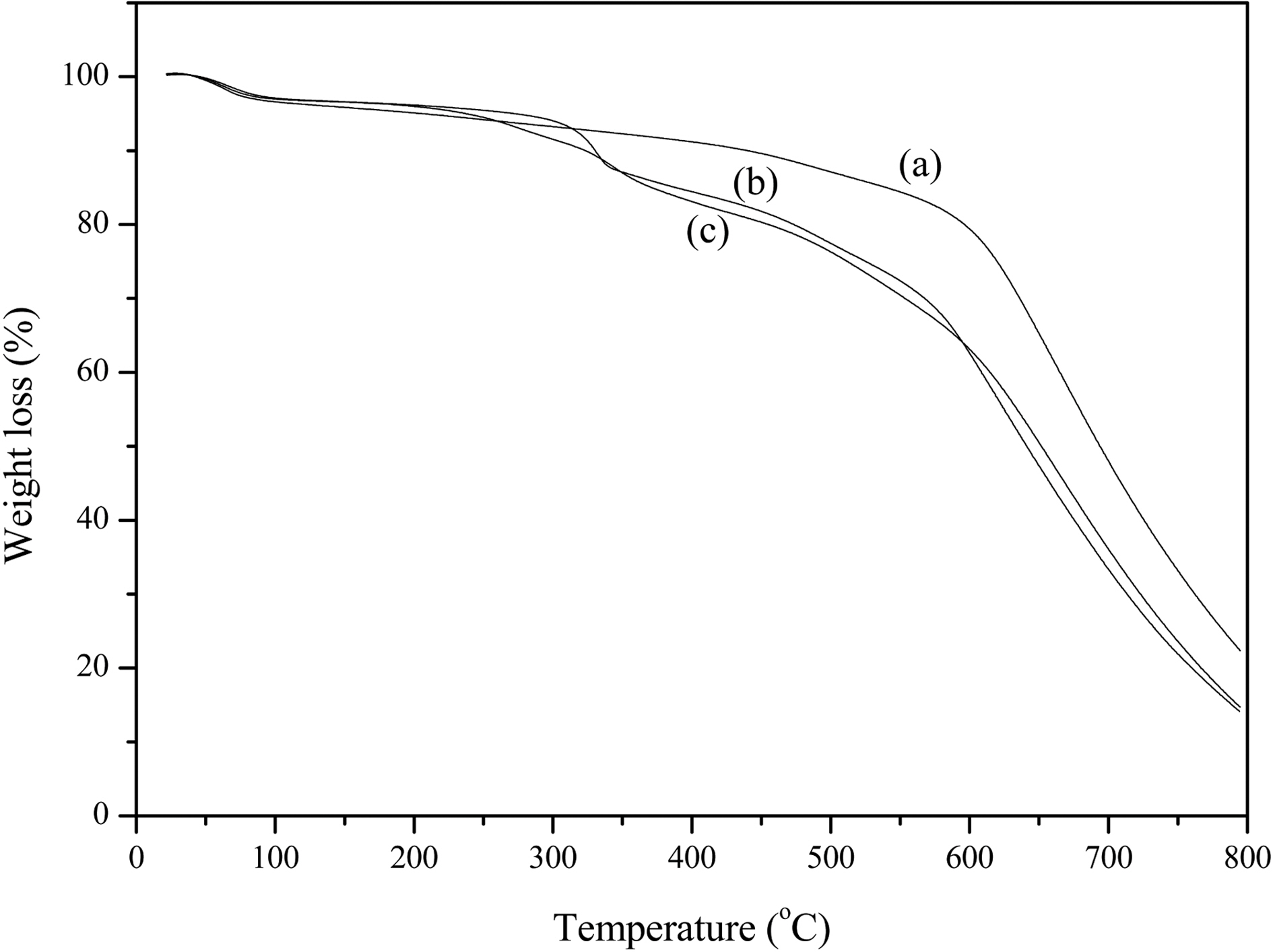
Fig. 4 shows the TGA curves of the purified MWNTs (a), ion-modified MWNTs (b), and IL-modified MWNTs (c) by one-step radiation graft polymerization. As shown in Fig. 4, the 1st weight loss (%) from 50℃ to 250℃ for vinyl polymer-graft MWNTs appeared due to moisture because of the hydrophilic properties of the grafted vinyl polymer. The 2nd weight loss (%) appeared in the range of 250~600℃ due to the grafted vinyl polymer weight loss. Results showed the graft yields (%) were in the range of about 20% after RIGP of vinyl monomers.
3.2. Tyrosinase-immobilized biosensor and its determing phenolic compound in commercial coffee
As mentioned above, ionic property (ion, IL)-modified
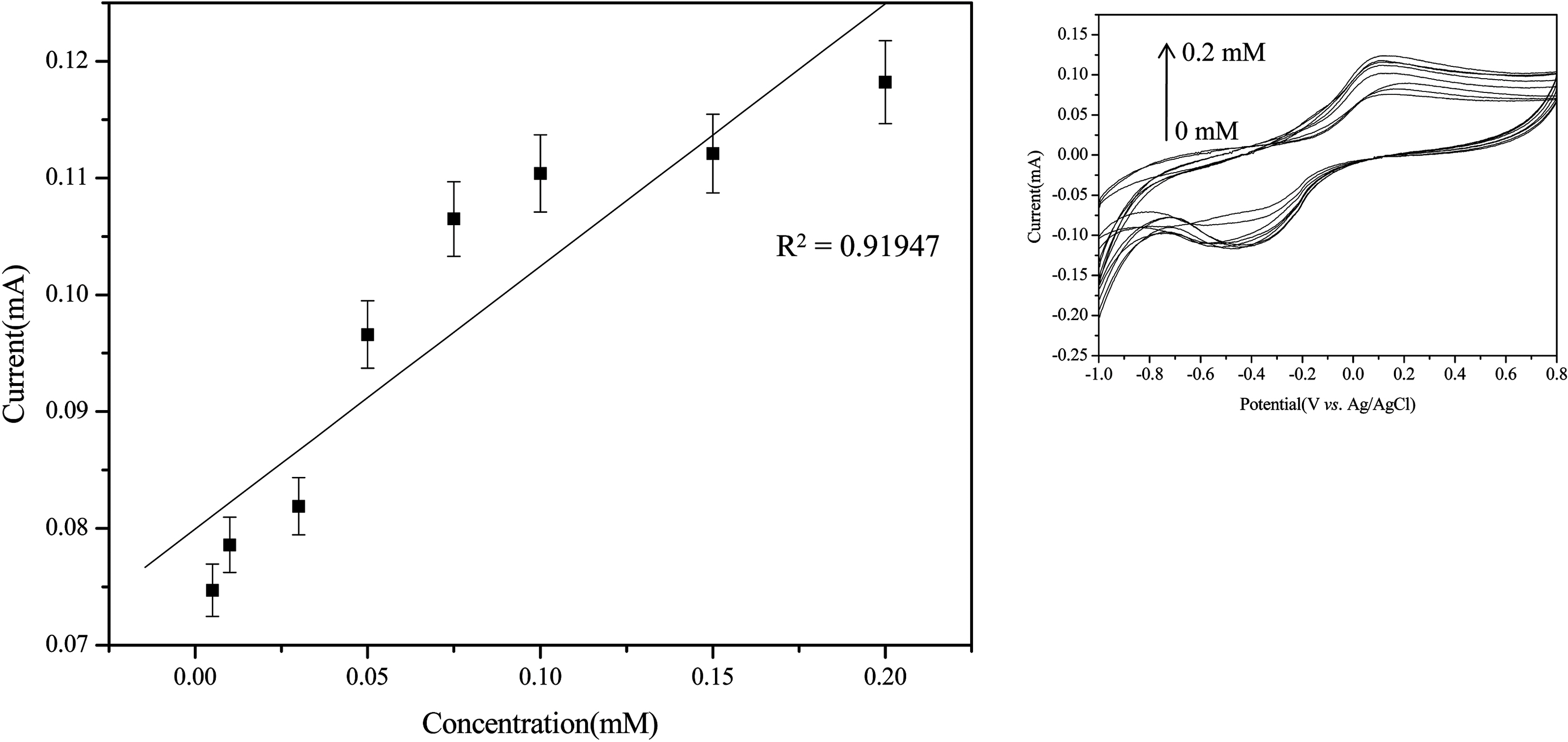
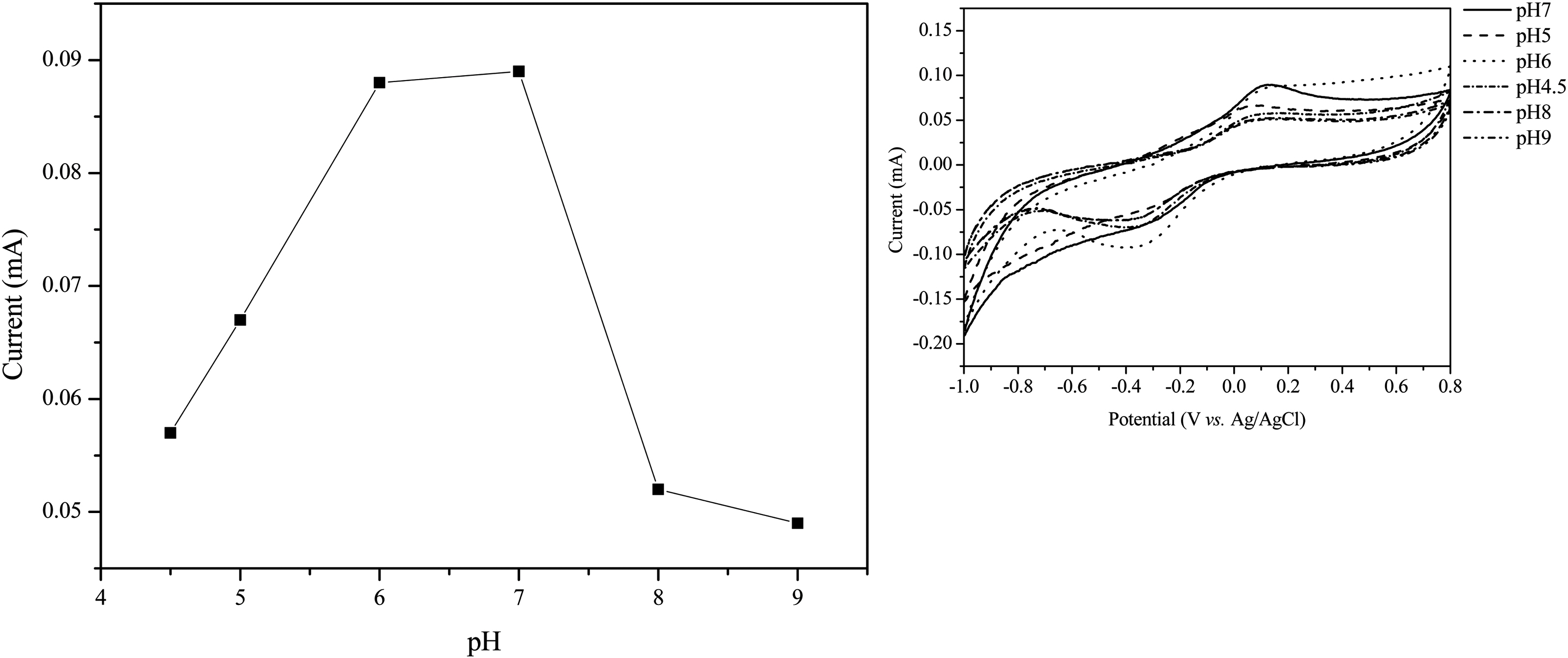
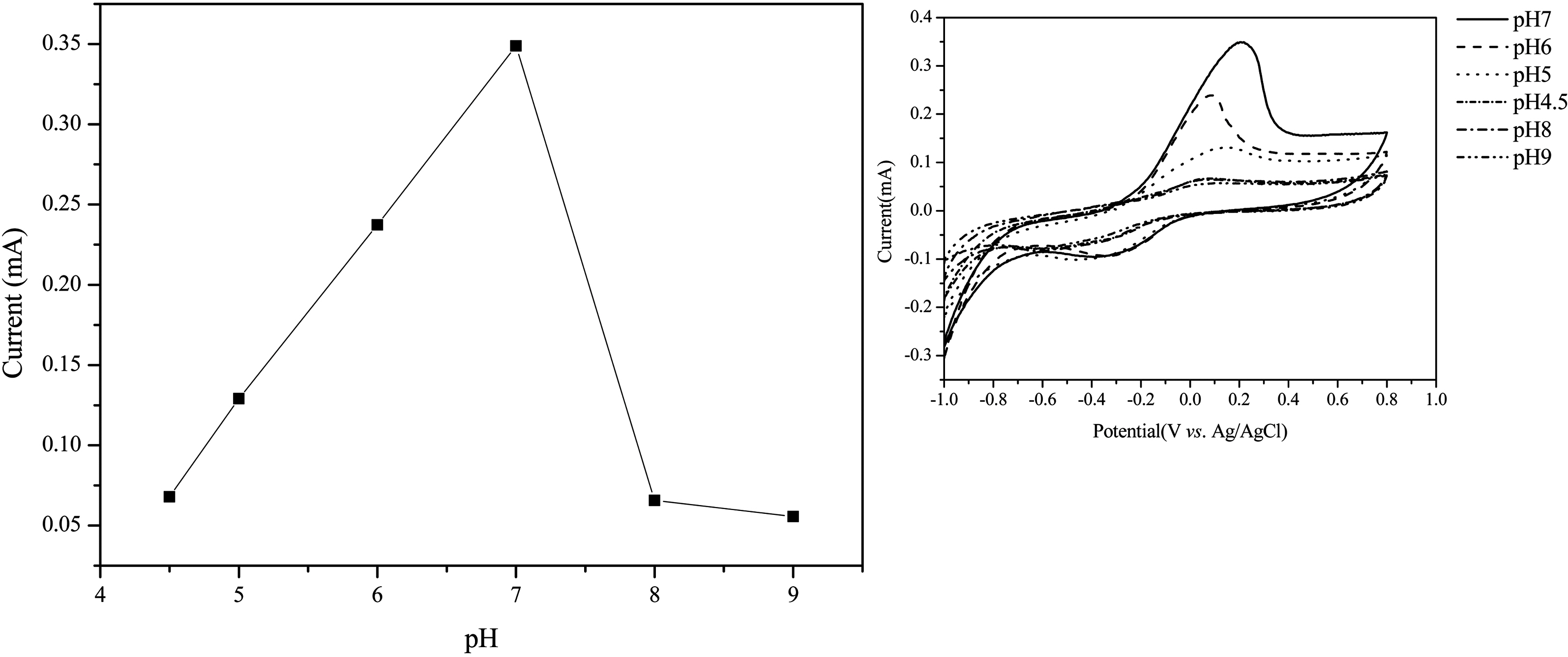
MWNTs can be used as supports for enzyme immobilization because of their pulse charge and improve sensitivity. In order to determine the sensitivity of the biosensor due to the ionic property-modified MWNTs electrode, we fabricated the ionic property-modified MWNTs electrode by the handcasting method as described same method [7,24]. Fig. 5 reveals SEM images of pure ITO surface (a),
In previous papers [7,24], the
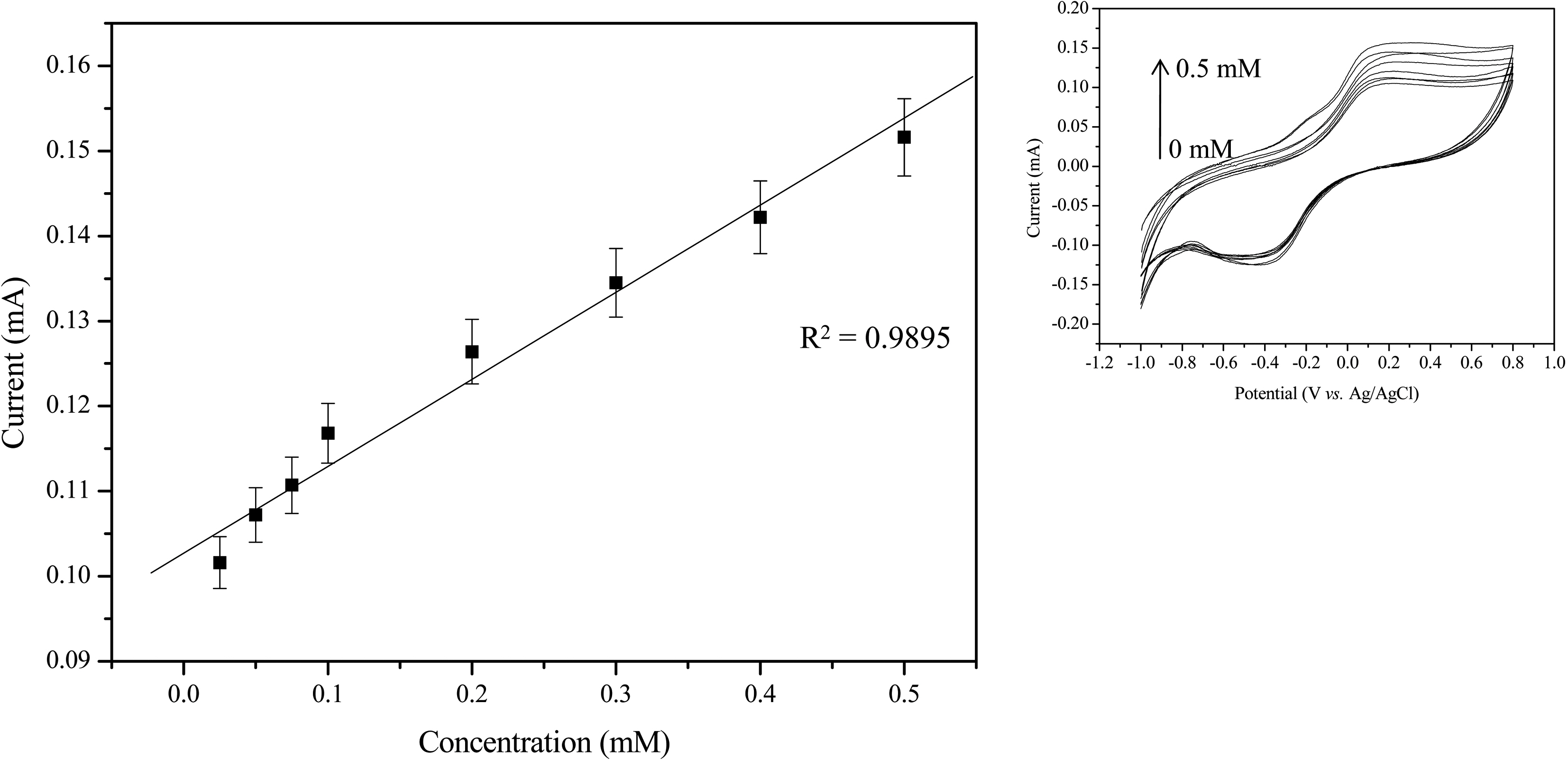
The electrochemical biosensing of phenols was performed under optimal experimental conditions. Another parameter affecting the sensing efficiency of the
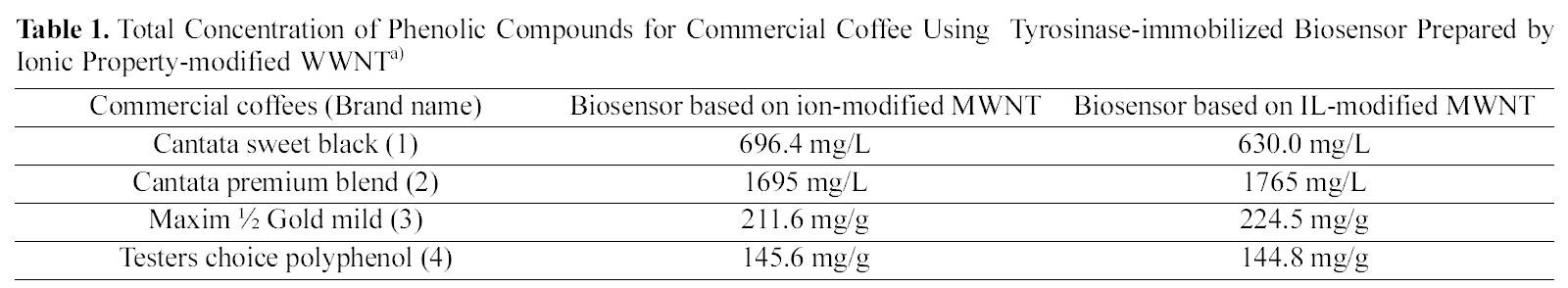
The commercial coffees such as Cantata sweet black (1) and Cantata premium blend (2) of the liquid type and Maxim ½ Gold mild (3) and Testers choice polyphenol (4) of the solid type were used in the analysis of phenolic compound(may be caffeine) amounts in coffee solution. Table 1
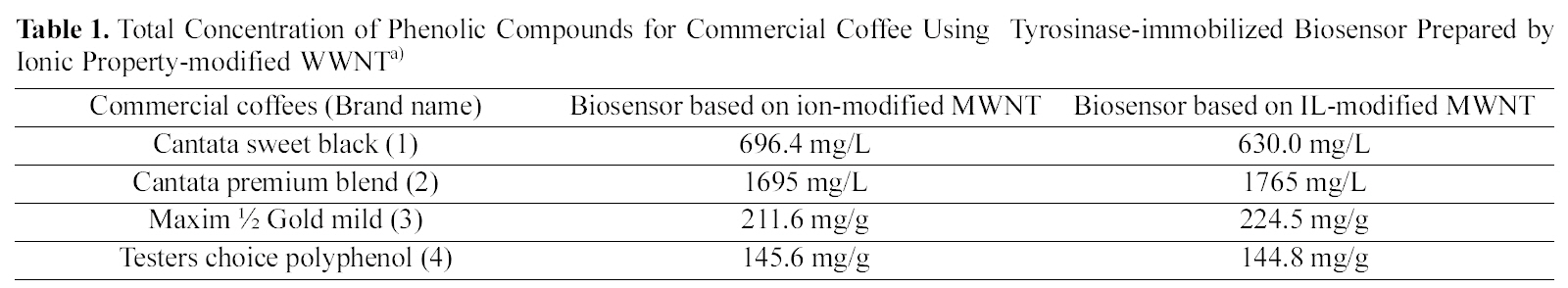
Total Concentration of Phenolic Compounds for Commercial Coffee Using Tyrosinase-immobilized Biosensor Prepared by Ionic Property-modified WWNTa)
summarizes total concentration of phenolic compounds for commercial coffee using
In this study, we fabricated a