Transdermal drug delivery (TDD) is convenient for delivery of therapeutic agents and can be a valuable alternative to oral administration which needs passage through the gastrointestinal tract. Advantages of the transdermal route include the circumvention of first-pass gut and liver metabolism, increased compliance, controlled plasma levels, and reduction of overall dose [1,2].
However, TDD has a limit in the extensive application due to the extremely low drug release rate from the matrix and the low permeability of drug through the skin. In general, the precise controls over the drug quantity and the release rate are required in order to optimize the drug therapy. TDD needs the drug carrier to respond and activate in a reproducible and predictable fashion under internal or external stimuli such as electric field [3], pH [4], and temperature [5]. The use of electric field as external stimulus seemed to be an efficient and simple method to increase the amount of released drug through the skin and to control the release of drug precisely [6].
Carbon nanotubes (CNTs) are the excellent material for the high technology applications where electrical properties are sought, due to their excellent electrical conductivity. CNTs were applied generally as reinforcing fillers for various nanocomposites due to their exceptional strength and high aspect ratio [7-10]. The bioactive materials endowed with immunological properties can be prepared by conjugating the bioactive peptides to the external walls of CNTs. CNTs are also applied to drug delivery [11] and biochemical sensing [12].
Hydrogels have been applied in the controlled drug delivery as the materials for coating or binding the pharmaceuticals [13,14]. The freezing-thawing method is one kind of methods to produce the hydrogels without the chemical cross-linkers [15,16]. Poly(vinyl alcohol) (PVA) hydrogels prepared by a freezing-thawing method are excellent candidates for biomaterials because they exhibit a high degree of swelling in water, an elastic nature, a nontoxicity,and biocompatibility [16].
In this study, the drug-loaded multi-walled carbon nanotube (MWCNT)/poly(vinyl alcohol) nanocomposite hydrogel films were prepared by a freezing-thawing method and these nanocomposite hydrogels were used as carrier matrix of model drug, coomassie brilliant blue. The morphology, compression modulus, and electrically modulated swelling and drug releasing behaviors of the nanocomposite hydrogels were investigated to develop the electro-responsive TDD.
PVA (Mw: 124,000-186,000, 99.0%) and MWCNTs were purchased from Sigma-Aldrich. The diameter of MWCNTs
[Table 1.] Classification of MWCNT/PVA nanocomposite hydrogels
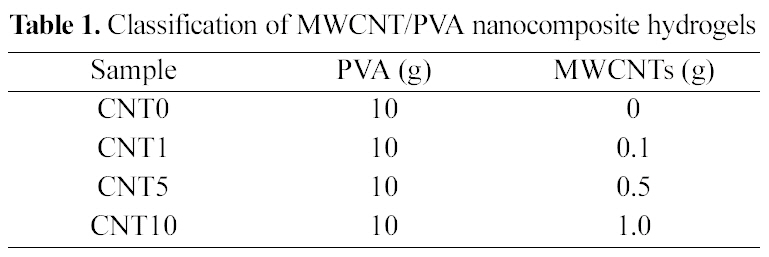
Classification of MWCNT/PVA nanocomposite hydrogels
is between 110 and 170 nm and the purity of MWCNTs is higher than 90%. Coomassie brilliant blue R-250 (CBB) (C47H50N3O7S2+) (λmax=583 nm), the model drug, was obtained from Biorad Laboratories.
2.2. Preparation of MWCNT/PVA nanocomposite hydrogel films
MWCNT/PVA nanocomposite hydrogel films were prepared by freezing-thawing technique. PVA solution(15 wt% w/v) was first prepared by dissolving PVA in deionized water for 6 h at 90℃. CBB was loaded into the 10ml of PVA solution under constant stirring after cooling the solution to room temperature. PVA/CBB solution was poured into a petri dish and then treated with the consecutive three cycles of freezing at ?70℃ for 1 h and thawing at 25℃ for 4 h to prepare the PVA hydrogel films containing CBB.
MWCNTs (1, 5, 10 wt%, w/w) were added in the 10ml of PVA solution followed by loading CBB with constant mechanical stirring. MWCNT/PVA/CBB solutions were treated with the same freezing-thawing cycles to prepare MWCNT/PVA nanocomposite hydrogel films containing CBB. The control MWCNT/PVA nanocomposite hydrogel film was prepared with the same procedure except loading CBB. The various MWCNT/PVA nanocomposite hydrogels are classified in Table 1.
Scheme 1. Schematic illustration of drug release experimental systems (a) Franz diffusion cell loaded with MWCNT/PVA nanocomposite hydrogels between copper cathode and nylon net and (b) Franz diffusion cell connected with the electric circuit.
The morphology of nanocomposites was studied with field-emission scanning electron microscopy (FE-SEM). FESEM was carried out at 15 kV using a Hitachi S-5500 microscope. Compression moduli were measured with universal testing machine (UTM, Instron 5583) using sample size of 20 mm×20 mm×7 mm with the rate of 1 mm/min.
The swelling behavior of nanocomposites was determined by a gravimetric method. The dried samples were immersed into the isotonic phosphate buffered saline (PBS, pH 7.4) for a certain period until the swelling equilibrium was reached under a constant voltage using power supply and then the swollen samples were taken out to remove excess of buffer solution on the surface by wiping with tissue paper. They were weighed immediately. The equilibrium swelling ratio(%) of the hydrogels was calculated as follows:
Swelling (%)=(Ws ?Wd) Wd×100,
where Wd and Ws are the weights of dry and swollen samples, respectively.
Permeation experiments were conducted in the vertical Franz-type diffusion cells (Disa, Milan). The nylon net was placed on the top of Franz diffusion cell containing the buffer solution as shown in Scheme 1. MWCNT/PVA nanocomposite hydrogels were placed between the copper cathode and the nylon net. The copper/hydrogel/net assembly was mounted on the receptor compartment. The resistance was used to keep the low current around 1 × 10-3 ~
1 ×10-4 mA as shown in Scheme 1 (b). The hairless mouse membrane was mounted between the donor and receptor compartments of the diffusion cells with the epithelial side facing the donor compartment. The diffusional surface area was 3.14 cm2. PBS (pH 7.4) was used for the receptor phase(15 ml) and maintained at 37 ± 1℃ using a circulating water bath. The receptor medium was mixed uniformly by magnetic stirring. After an equilibration period of 30 min with PBS on both sides of the membrane tissue, 0.2 g of the nanocomposite hydrogel film was applied to the mucosal surface in the donor compartment and 0.5 ml of PBS was then added followed by applying the electric voltage (0, 1, 5, and 10 V) using DC power supply (Itech, IT6721). PBS(2 ml) containing the released drug was withdrawn from the receiver compartment periodically and replaced immediately with an equivalent volume of fresh PBS at 37±1℃. The PBS samples were assayed by a UV spectrometer at 583 nm [17].The amount of released CBB was determined by a cumulative curve.
3.1. Morphology of MWCNT/PVA nanocomposite hydrogels
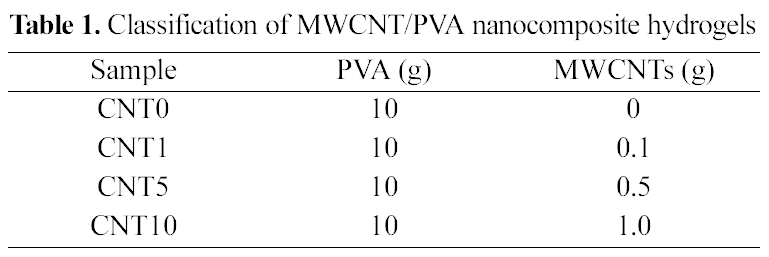
SEM images of MWCNT/PVA nanocomposite hydrogels are presented in Fig. 1 to Fig 3. Fig. 1 shows the cross sectional morphology of MWCNT/PVA nanocomposite hydrogels. MWCNTs were found in the hydrogel matrices without any crevice between hydrogel and MWCNTs. The good contact at the interface contributes an important role in the electrically modulating performance by minimizing the interfacial electrical resistance. The increased number of
embedded MWCNTs was observed in the nanocomposites with higher amount of MWCNTs loaded as shown in Fig. 1.
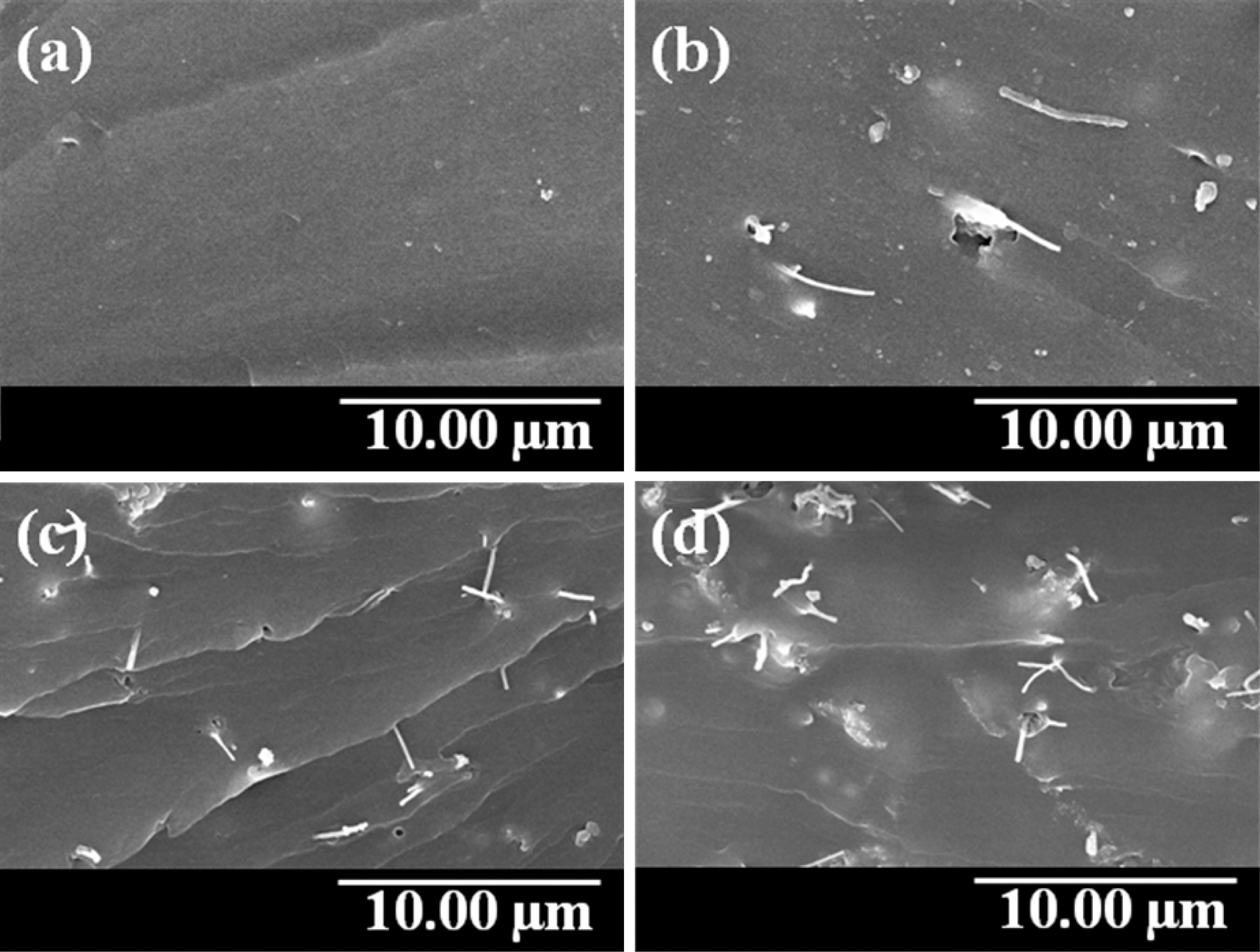
Fig. 2 and Fig. 3 show the morphology of freeze-dried MWCNT/PVA nanocomposite hydrogel films after swelling.Fig. 2 represents the variations in surface morphology of MWCNT/PVA nanocomposites depending on the content of MWCNTs at 10. On the other hand, Fig. 3 shows the variations in surface morphology of CNT5 nanocomposite depending on the applied voltages. MWCNT/PVA nanocomposites swelled larger and showed the bigger pore size as the content of MWCNTs increased as shown in Fig. 2. However, CNT5 and CNT10 showed the similar pore size under same condition revealing the threshold for swelling. MWCNT/PVA nanocomposites also swelled higher and showed the
larger pore size as the applied voltage increased due to their electro-sensitive properties as shown in Fig. 3.
3.2. Compression moduli of MWCNT/PVA nanocomposite hydrogels
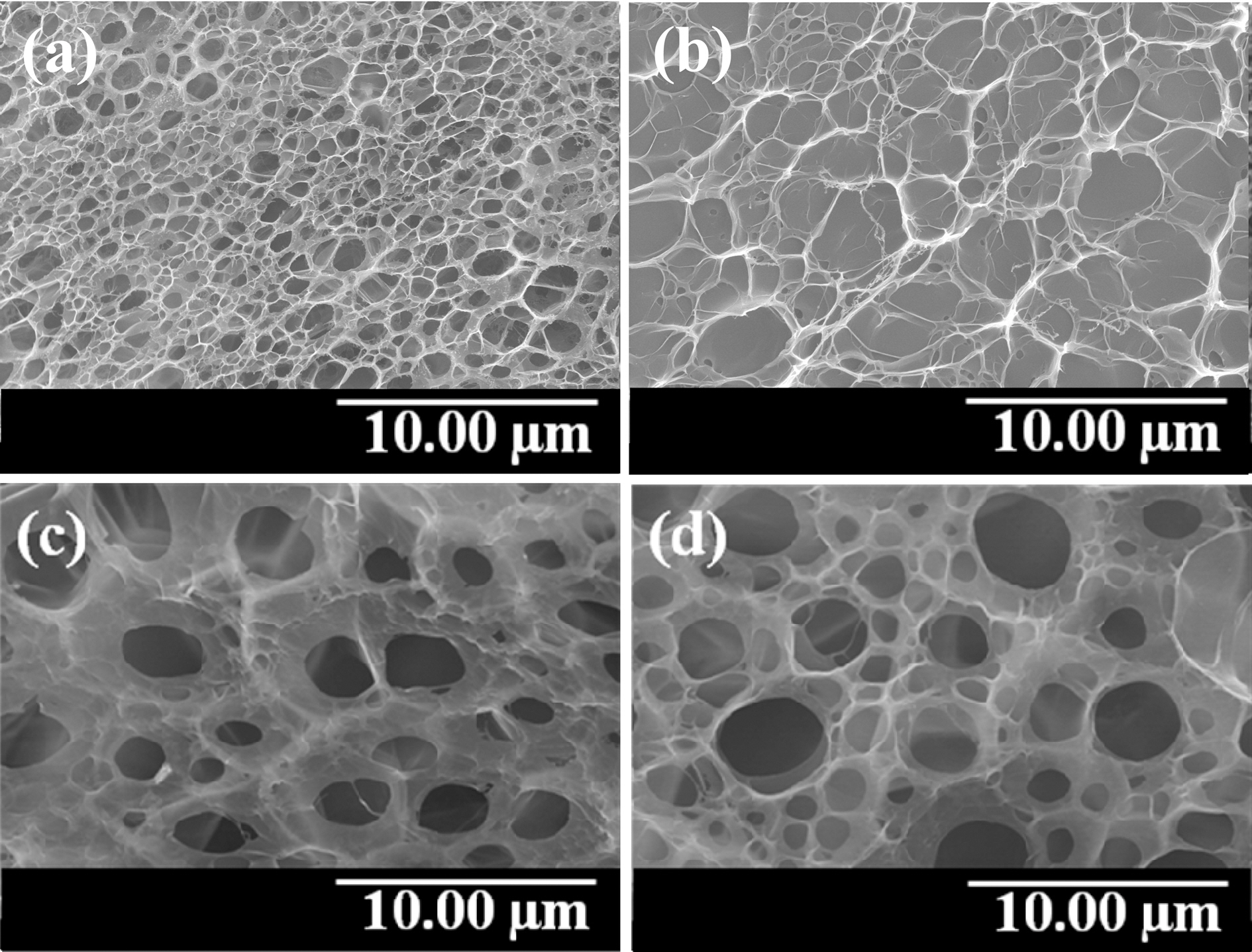
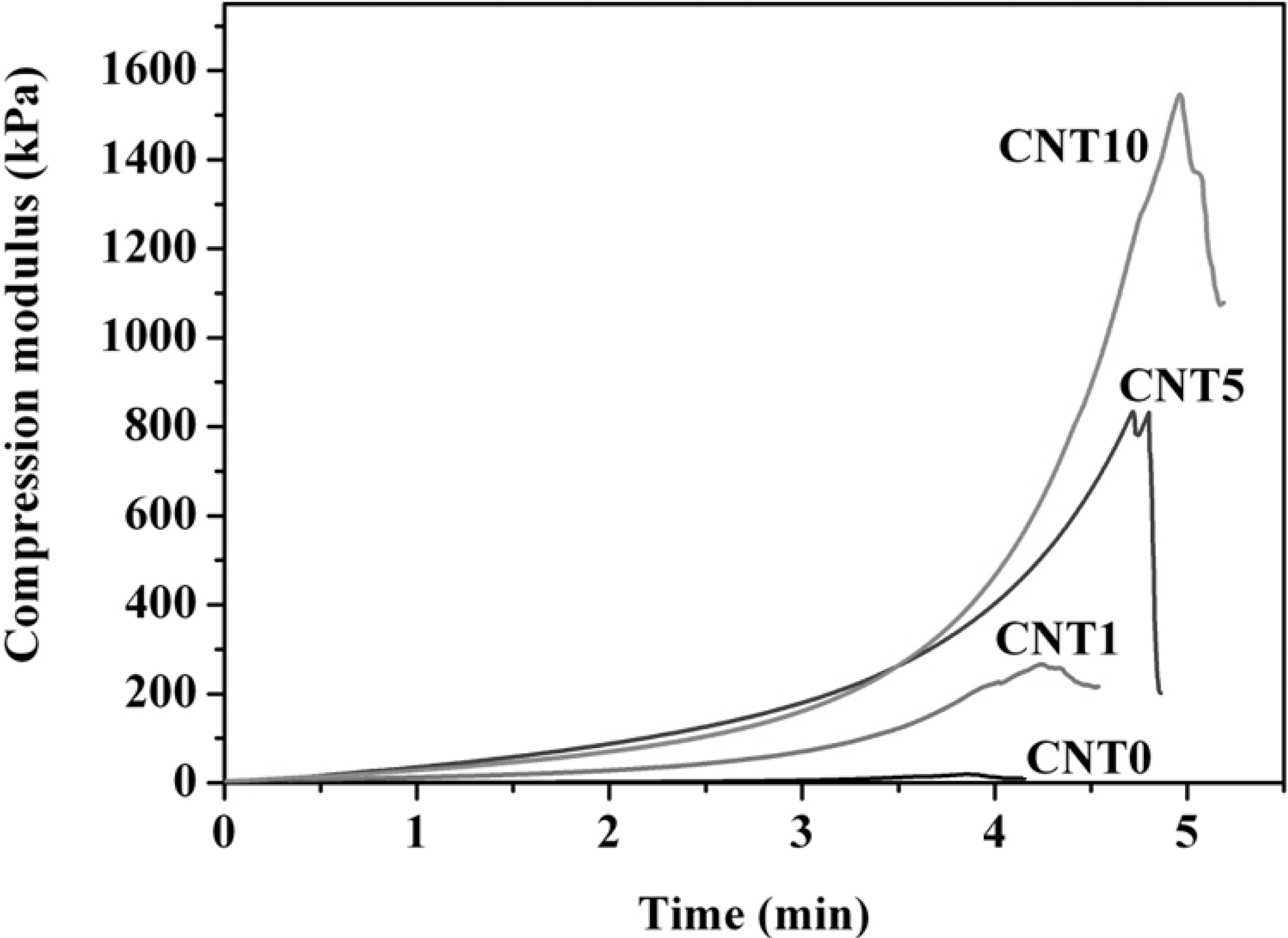
Fig. 4 represents the compression moduli of MWCNTs/PVA nanocomposite hydrogels. The compression moduli increased greatly from 40 to 1500 kPa using higher content of MWCNTs. MWCNTs worked effectively to improve the mechanical properties of nanocomposite hydrogels due to the good contact at the interfaces.
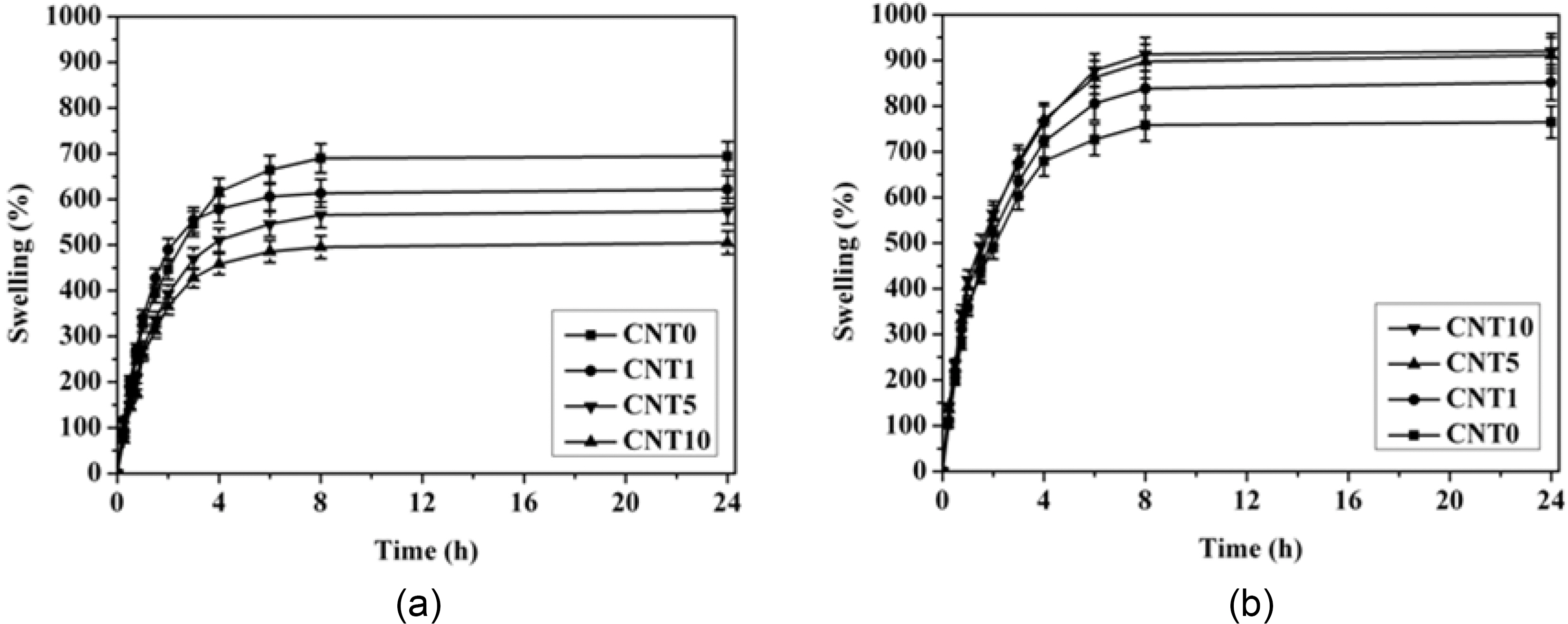
3.3. Swelling behavior of MWCNT/PVA nanocomposite hydrogels
The swelling behavior of MWCNT/PVA nanocomposite hydrogels is shown in Fig. 5. CNT0, which did not contain MWCNTs, showed the highest swelling ratio and the swelling ratio of MWCNT/PVA nanocomposite hydrogels decreased as the content of MWCNTs increased when the electric voltage was not applied to the nanocomposites as shown in Fig. 5(a). MWCNTs worked only as reinforcing ingredient having good interfacial contact to increase the physical crosslinking density of hydrogels under the electric voltage of 0 V. The additional physical crosslinking resulting from the favorable interaction between MWCNT and hydrogels contributed partly to the reduction of swelling of nanocomposites.
On the other hand, the swelling behavior of nanocomposites was totally different under the electric voltages applied to the nanocomposites. The swelling ratio of MWCNT/PVA nanocomposite hydrogels increased as the content of MWCNTs increased when the electric voltage was applied to the nanocomposites as shown in Fig. 5(b). MWCNTs made the efficient pathway of electric field due to their own electrical conductivity. Hence, MWCNTs contributed a large share in increasing the swelling ratio of nanocomposites by accelerating the ionization of functional groups in the hydrophilic hydrogel matrices under electric voltage applied [18].
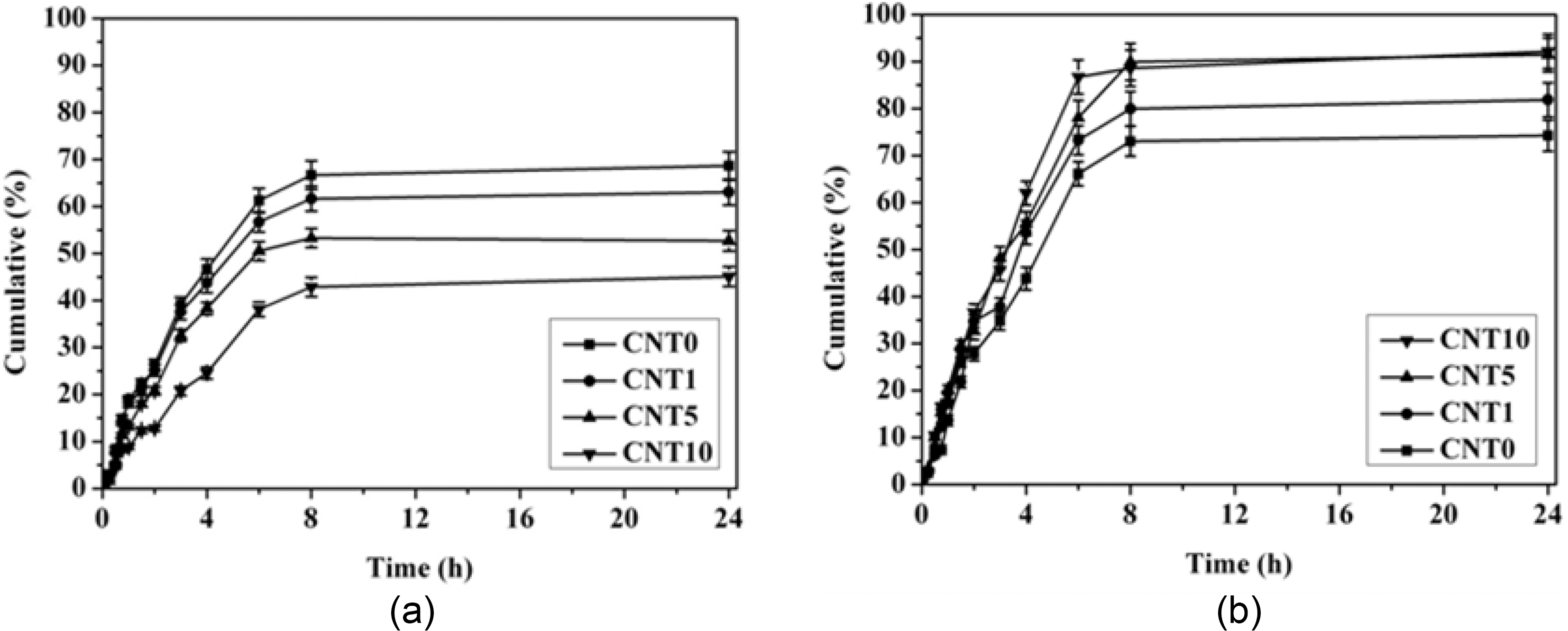
3.4. Electro-responsive release behavior of MWCNT/PVA nanocomposite hydrogels
The drug release behavior of MWCNT/PVA nanocomposite hydrogels is shown in Fig. 6. The drug release behavior of MWCNT/PVA nanocomposites depends strongly on both the content of MWCNTs and the applied electric voltages. The cumulative amount of released drug and the release rate decreased as the content of MWCNTs increased at 0 V due to the lower swelling of hydrogels. On the other hand, both the cumulative amount of released drug and the release rate increased with the higher content of MWCNTs at 10 V as predicted from the swelling behavior of nanocomposites displayed in Fig. 5. Hence, the electro-responsive swelling behavior of hydrogels was a main factor for the electrosensitive controlling of drug release from the MWCNT/PVA nanocomposites.
MWCNT/PVA nanocomposite hydrogel films were prepared by a freezing-thawing method for the electroresponsive transdermal drug delivery. MWCNTs were uniformly dispersed in the hydrogel matrices with good contact between hydrogel and MWCNTs resulting in the minimization of interfacial electrical resistance. Both the pore size and the swelling ratio of hydrogel matrices increased under applied electric voltages due to the accelerated ionization of functional groups in the hydrophilic hydrogel matrices as the content of MWCNTs increased in the nanocomposites. The compression moduli of hydrogels increased 38 times higher by incorporating higher content of MWCNTs. The electro-responsive drug releasing behavior of MWCNT/PVA nanocomposite hydrogels depended on the content of MWCNTs and the applied electric voltage because the electro-sensitive swelling behavior of nanocomposites played a decisive role in drug release characteristics. The electro-responsive MWCNT/PVA hydrogels could be applied in the programmable delivery of drugs and the faster delivery of cosmetic ingredients through the skin.