Carbon fiber-reinforced carbon (C/C) composites have garnered much attention from researchers due to their outstanding mechanical properties including high strength-to-weight ratio, low density, excellent thermal stability, high thermal conductivity, low coefficient of thermal expansion, and resistance to thermal shock and ablation [1-3]. Furthermore, C/C composites are considered promising candidates for such applications as brake discs in advanced aircrafts, heat shields for reentry vehicles, nozzles in solid rocket motors, and nose tips and leading edges in aerospace applications [4,5]. However, the application of C/C composites has been limited because they begin to oxidize at temperatures greater than 450℃ [6]. Thus, to overcome this limitation, the oxidation stability of C/C composites must be improved.
Two approaches have generally been used to improve the oxidation stability of these composites. One approach entails coating the carbon fibers or the composite with an antioxidant layer, whereas the other involves the direct addition of antioxidant fillers into the matrix [7]. Accordingly, ceramic materials such as SiO2, MoSi2, ZrB2, SiB4, and Si3N4 have been used to improve the oxidation stability of the composites [8,9]. However, these ceramic fillers are expensive and can adversely affect human health [8].
In this regard, it is worth considering the use of layered silicates (clays) in C/C composites. Clays have drawn much interest both in academia and industry, because they can remarkably improve the mechanical properties of composite materials when used as fillers, particularly in clay/polymer nanocomposites [10]. Similarly clays can be used in C/C composites, another important
class of composite materials.
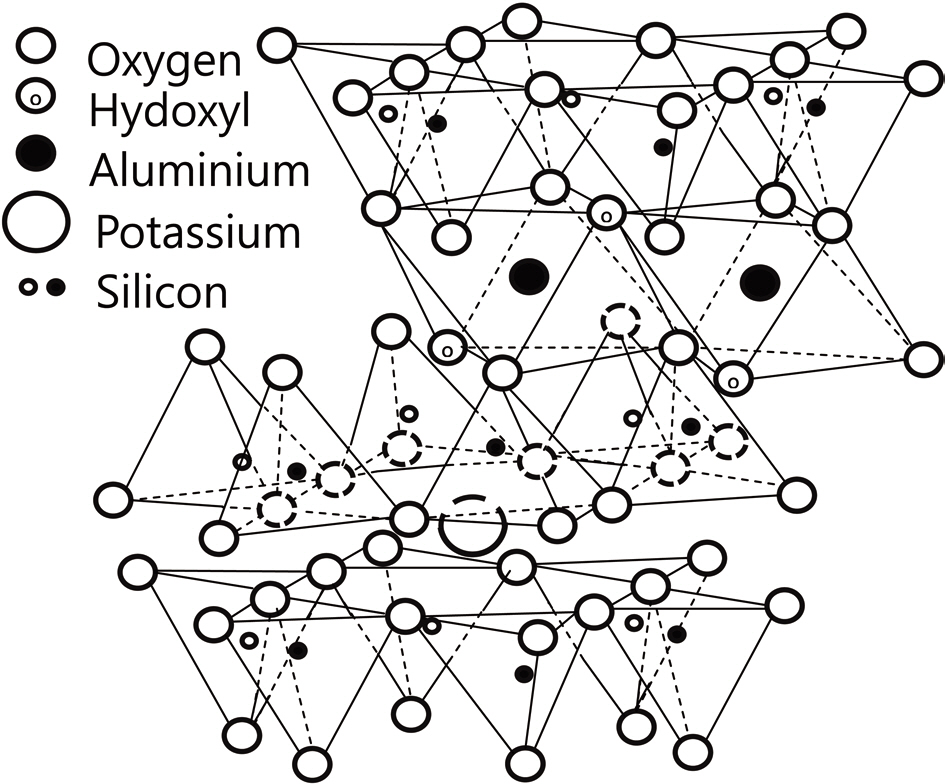
Among clay materials, illite is one of the most abundant on earth, and is therefore a promising clay filler candidate. The chemical structure of illite is shown in Fig. 1. Pure illite contains two SiO2 tetrahedral sheets covering a central Al2O3 octahedral sheet, whereas the raw illite used in this study contained quartz and kaolinite, both mainly consisting of SiO2, as impurities [11]. SiO2 and Al2O3 are widely used fillers that are employed to provide good thermal properties [12]. Moreover, it is possible to form SiC via the reaction between illite, phenol resin, and carbon fiber during the carbonization of phenol resin; this results in additional improvement in the oxidation stability of C/C composites, as noted in our previous report [13]. Additionally, as shown in Fig. 1, illite has a layered structure, which is a characteristic feature of solid, high-temperature lubricants used to enhance the impregnation or infiltration of liquid materials, such as Si liquid slurries and polymers or pitch melts, by reducing abrasion [14].This enhancement was also expected to improve the oxidation stability and mechanical properties of C/C composites.
Our previous studies showed that when using illite as a filler, both the oxidation stability and the mechanical properties of C/C composites were improved. Furthermore, the oxidation stability of the composites was also improved after physically modifying illite in the pursuit of possible novel applications of illite in C/C composites [13,15]. Hence, in this study, to further investigate illite’s potential use in C/C composites, illite was added to a phenol resin carbon matrix precursor, and the mixture was impregnated only once to emphasize both the effect of the illite addition and heat treatment at different temperatures. The effects of the heat-treatment temperature on the chemical structure, microstructure, and thermal oxidation properties of the resulting illite-containing C/C composites were investigated.
Illite (1000 mesh) was provided by Yonggoong Illite Co. (Youngdong, Korea). A needle-punched carbon preform was manufactured with 12 K oxy-PAN fibers and was provided by DACC Co., Ltd. (Jeonju, Korea). Resol-type phenol resin (HM2) was purchased from Kolon Chemical Co. (Gimcheon, Korea).
Illite-containing C/C composites were prepared in the following manner. Illite was added to the phenol resin at 0 and 10 wt% of the phenol-resin base and stirred for 2 h. A needle-punched carbon preform was then impregnated with the illite/phenol
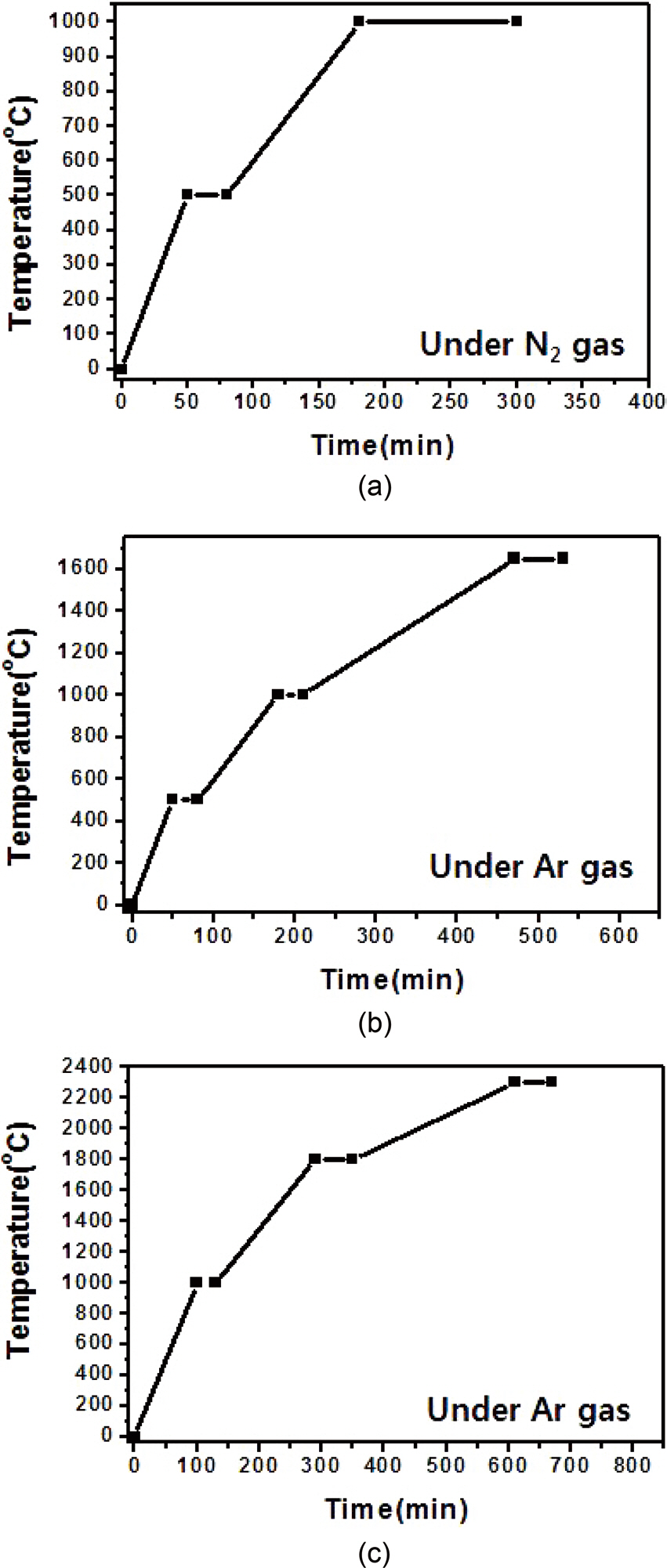
resin mixture at 0.2 bars for 10 min. The impregnated preform was dried in a thermoreactor at 70℃ for 3 h. The phenol-resinimpregnated carbon preform was cured at 130℃ for 5 h. Finally, the phenol resin/carbon composite was carbonized at 1,000, 1,650, or 2,300℃ using the procedure illustrated in Fig. 2.
2.3 Characterization of the prepared C/C composites
X-ray diffraction experiments (XRD, D/MAX-2200 Ultima/PC; Rigaku International Corporation, Japan) were performed to investigate the chemical changes in the C/C composites (in particular, the formation of SiC) in response to different heat-treatment temperatures. Scanning electron microscopy (SEM, JSM 700F; JEOL Co., Japan) was used to observe the effect of different heat-treatment temperatures on the microstructures of the prepared C/C composites. The bulk densities and porosities of the prepared composites were evaluated using the ASTM C20 method. A thermogravimetric analysis (TGA, TGA/SDTA851; Mettler-Toledo, Switzerland) was performed to evaluate the thermal oxidation stability of the prepared samples at a heating rate of 5℃/min in air.
3.1 Effect of the heat-treatment temperature on the chemical structures of the prepared composites
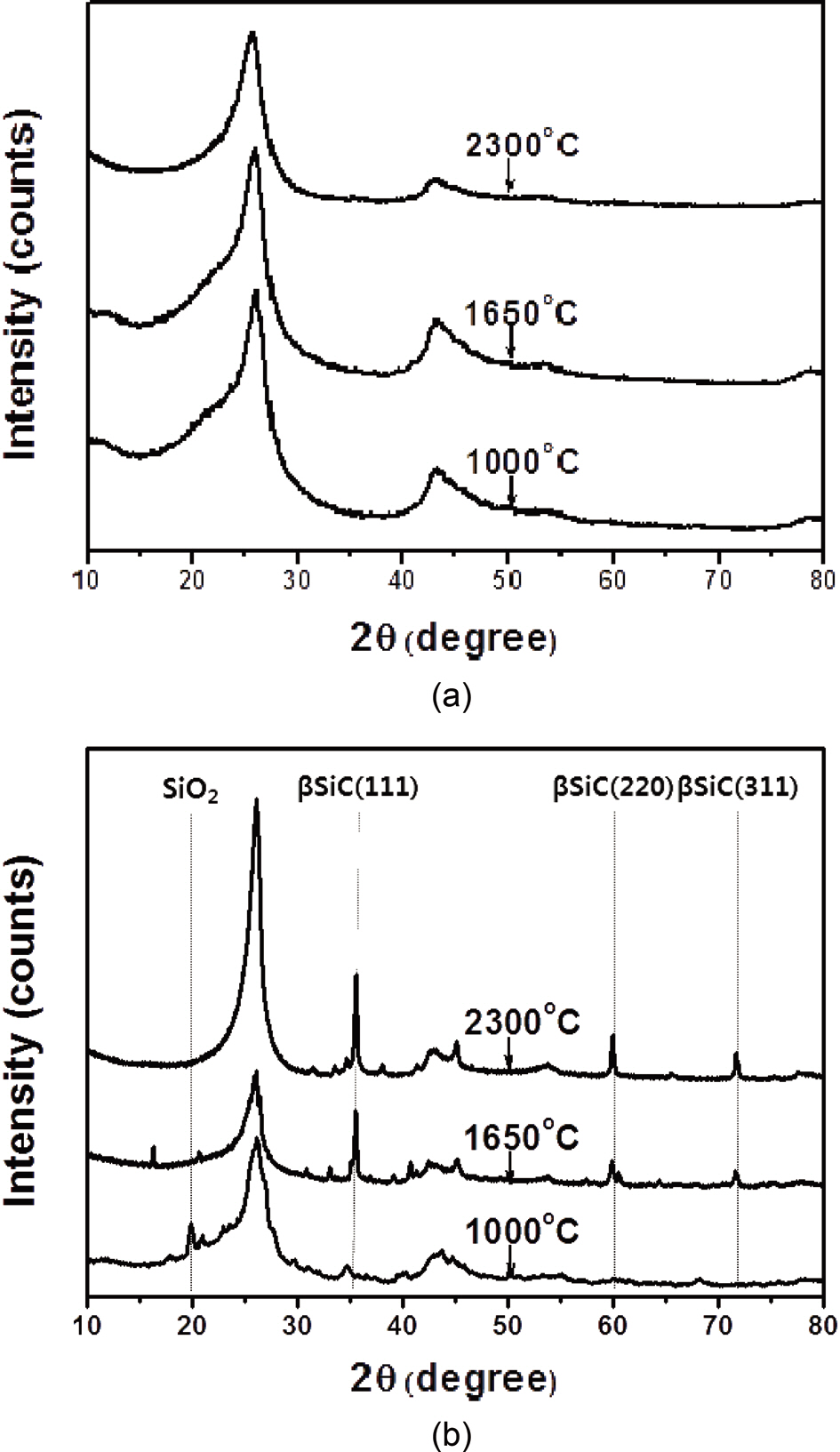
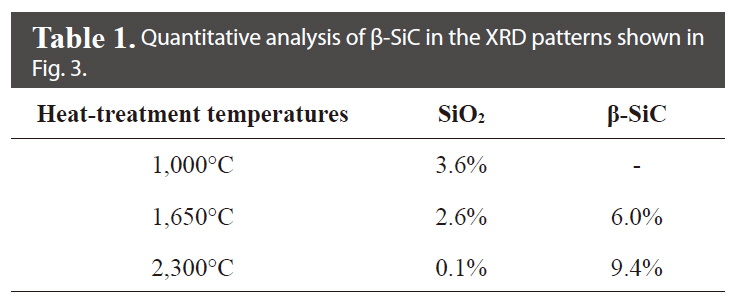
Fig. 3 shows the XRD patterns of the illite-containing C/C composites prepared with and without illite at different heat-treatment temperatures. In Fig. 3a and b, the peak at 26°, resulting from the (0 0 2) phase of the crystalline carbon, was observed in all XRD patterns of the prepared composites, and the peak became sharper as the heat-treatment temperature was increased due to the increased crystallinity of the carbon materials. With a heat-treatment temperature of 1,000℃, an SiO2 peak at 19.88°, characteristic of the illite, was observed without any SiC peak. At heat-treatment temperatures of 1,650 and 2,300℃, three peaks were observed, at 35.68, 60.06, and 71.80°, corresponding to the (1 1 1), (2 2 0), and (3 1 1) peaks of β-SiC, respectively; these peak positions are in good agreement with the findings of other studies [16-18]. β-SiC was formed as a product of the carbothermal reaction as a result of intimate contact between the SiO2 in the illite and the phenol resin in the precursor or carbon fibers in the preform [13,15]. It was also observed that the β-SiC peaks became sharper as the heat-treatment temperature was increased. Furthermore, as shown in Table 1, it was observed that the formation of β-SiC in the illite-containing C/C composites increased by approximately 57% when the heat-treatment temperature was increased from 1650℃ to 2300℃. It was also observed that the SiO2 peak essentially disappeared at the heat-treatment temperature of 2300℃, suggesting that most of the illite had reacted to form SiC.
Other new, small peaks at 33.84, 41.34, and 56.10° were also observed in the sample patterns. These peaks corresponded to α-SiC.According to a computer simulation by Pujar and Cawley [19], these peaks arise from stacking faults, which are crystallographic defects in the stacking sequence [17]. Another study reported that these peaks arise from the presence of the 2H polytype [20], which
[Table 1.] Quantitative analysis of β-SiC in the XRD patterns shown in Fig. 3
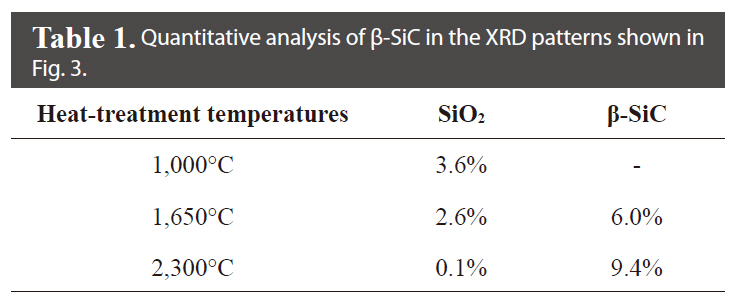
Quantitative analysis of β-SiC in the XRD patterns shown in Fig. 3
is composed of two planar elements stacked alternatively, inducing a much stronger reflection peak near 38.3°. However, that peak was not observed in the XRD patterns of the samples, indicating that these peaks resulted from stacking faults.
3.2 Effect of heat-treatment temperature on the microstructures of the prepared composites
The microstructures of the prepared composites are shown
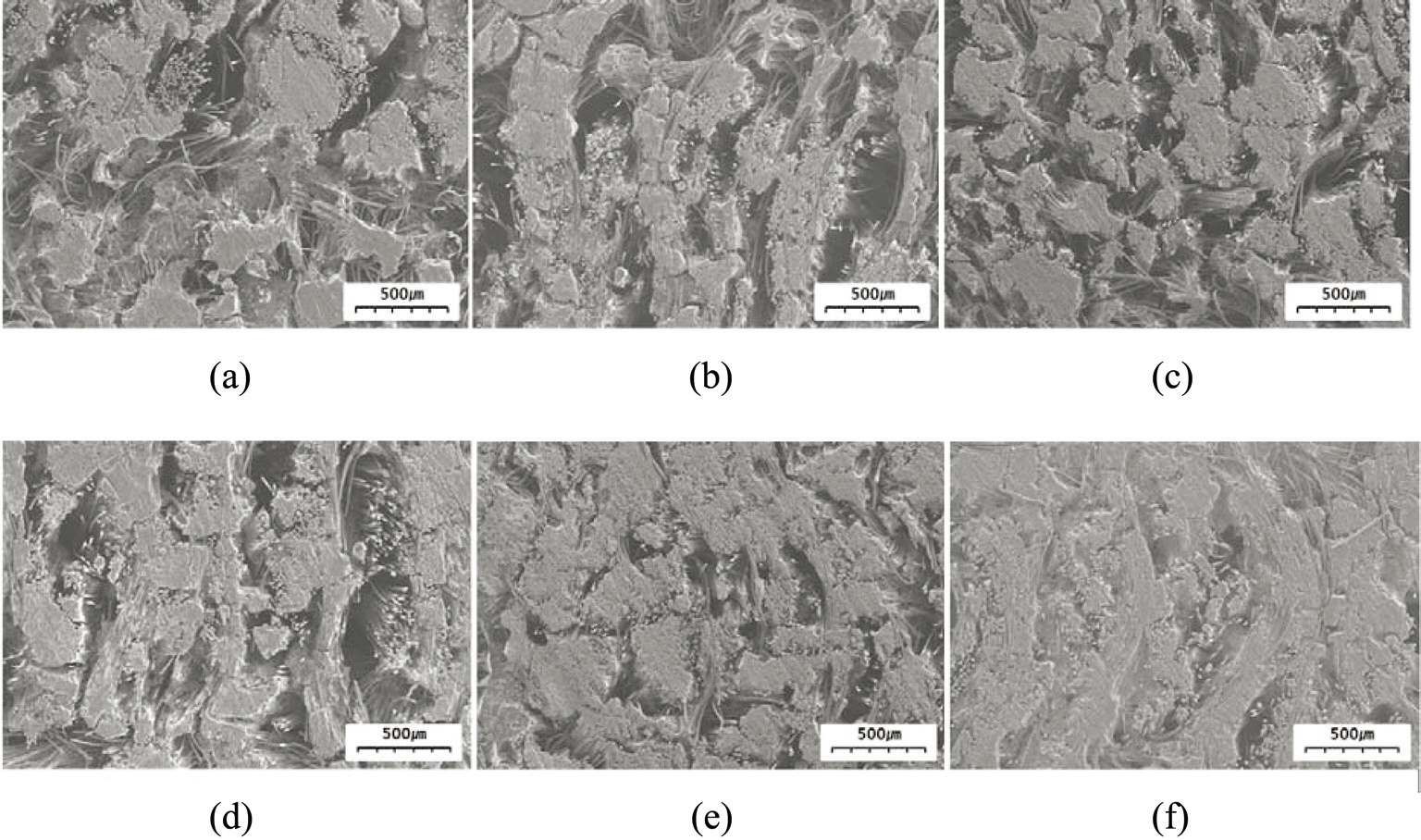
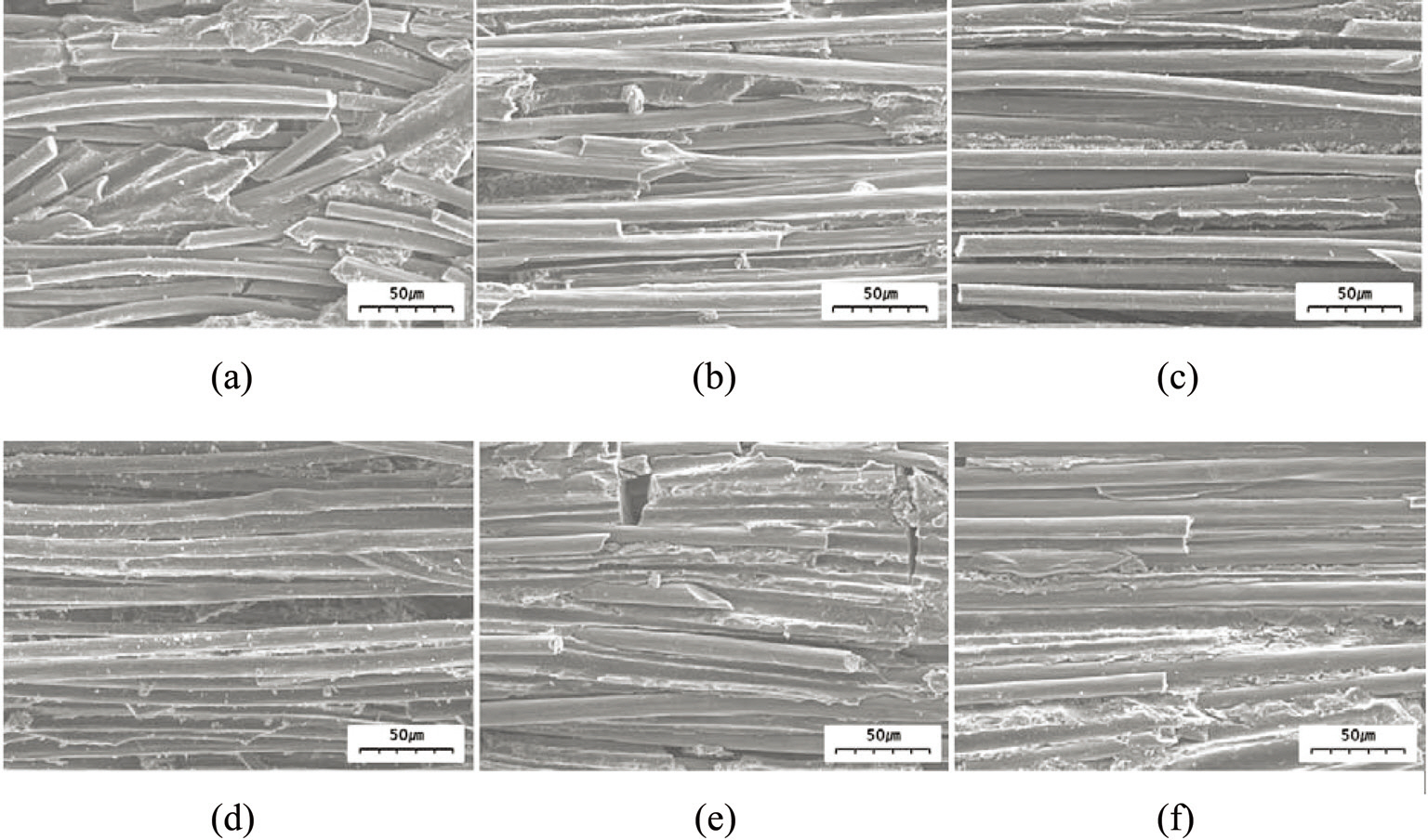
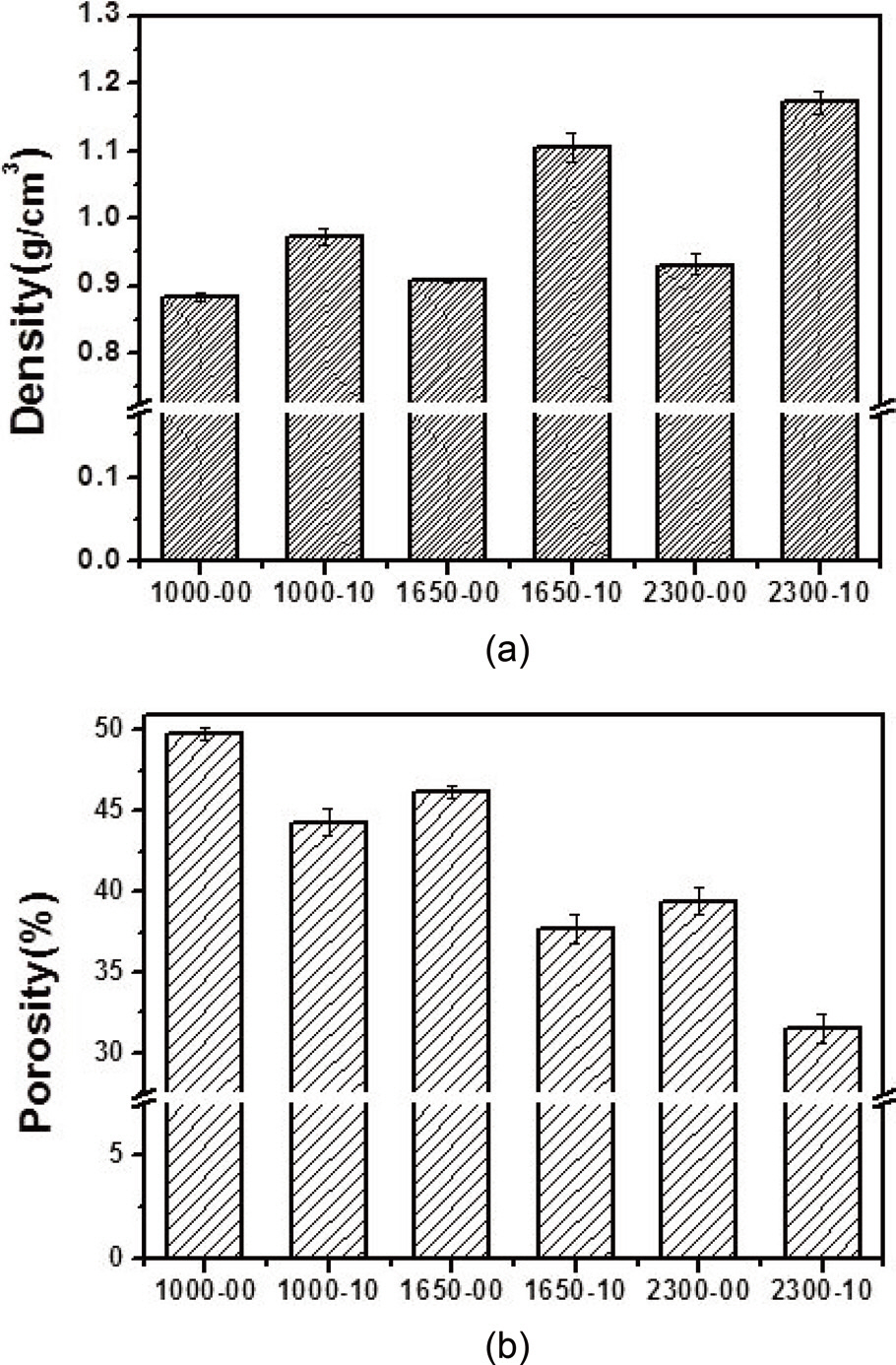
in Figs. 4 and 5. At the lowest magnification (50×), it can be observed in the SEM images in Fig. 4 that the C/C composites with illite (Fig. 4d-f) contained fewer pores than those without illite (Fig. 4a-c) when prepared at the same heat-treatment temperature. At higher magnification (500×), the SEM images in Fig. 5 show that the composite without illite contained more voids between its constituent fibers and more interfacial cracks between its fiber bundles and matrix (Fig. 5a-c) compared with the composite incorporating illite (Fig. 5d-f). Comparison of each set of two composites prepared at the same heat-treatment temperature also suggests that the composites with illite were denser than those without illite (Fig. 5). These observations were confirmed by measuring the bulk densities and the porosities of the composites using the ASTM C20 method; the results are shown in Fig. 6. The composites with illite exhibited bulk densities 11-20% higher than those without illite, as shown in Fig. 6a, whereas the composites with illite exhibited porosities 10-18% lower than those without illite, as shown in Fig. 6b.
These results may be attributed to the lubricating effect of illite. Illite is a silicate with a layered structure, as shown in Fig. 1. Layered materials, such as graphite, boron nitride, molybdenum disulfide,and even clay-based talc, are often used as solid lubricants
[14,21,22]. Therefore, illite may reduce the abrasion between the liquid phenolic resin and the carbon preform [22], enhancing the impregnation of the phenol resin into the carbon preform. This enhanced impregnation would result in fewer pores, voids, and interfacial cracks in the composites and subsequently lead to composites with greater bulk densities and lower porosities. Here, it is important to note that the densities of the composites also increased as the heat-treatment temperature was increased. This increase was due to the increase in crystallinity of the carbon material with increasing heat-treatment temperature [23].
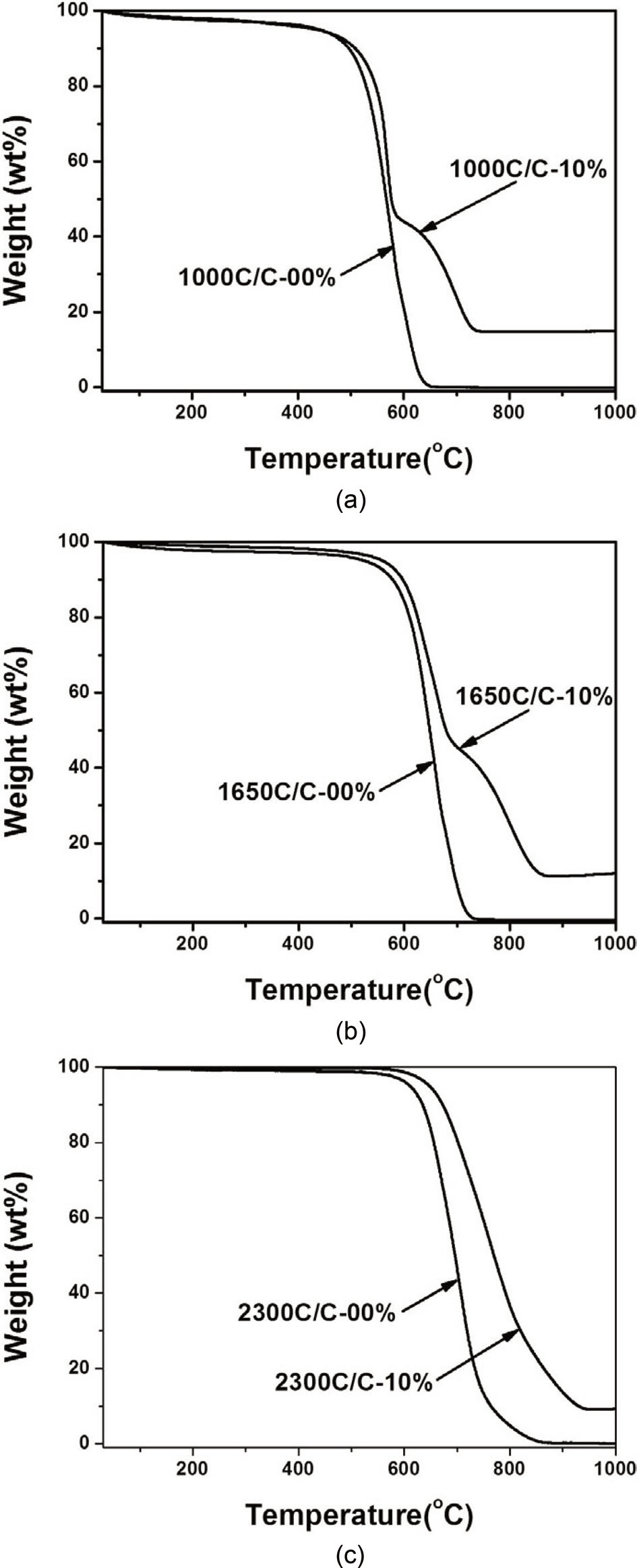
Fig. 7 illustrates the thermal oxidation stability of the prepared composites in air. As the heat-treatment temperature was increased, the initial decomposition temperature (IDT) by thermal oxidation also increased due to the increasingly crystalline structure of the carbon material [23]. At the same heat-treatment temperature, the IDT was higher in the composites with illite than in those without illite. For example, at the heat-treatment temperature of 2,300℃, the IDT of the composite with illite increased by approximately 30℃ over the corresponding compos-
ite without illite, from 613℃ to 643℃. This is primarily due to increased SiC formation induced by the higher heat-treatment temperature. When the composite was prepared at 2,300℃, almost all of the illite reacted to form SiC, which is highly resistant to thermal oxidation. Therefore, the IDT significantly increased compared with the other composites with illite.
It is also important to note that the oxidation behavior of the composite prepared at 2,300℃ was different from that of the other composites. With heat-treatment temperatures of 1,000 and 1,650℃, shoulders were observed in the TGA curves of the composites, whereas shoulders were not observed in the TGA curves of the composites when the heat-treatment temperature was 2,300℃. We have previously reported that these shoulders suggest a delay of oxidation and are due to improved fibermatrix interfacial adhesion, which results in fewer pores, voids,and cracks and thus hinders the diffusion of oxygen within the composite [13,15]. However, no shoulders were observed when the composite was heat-treated at 2,300℃, at which all the illite reacted to form SiC. Hence, the shoulder and the subsequent delay of oxidation may be attributed to the absorption of energy by the illite. It is also possible that the illite absorbed energy during exfoliation, as it has been previously reported that illite does not decompose at temperatures less than 1,000℃ in air [13].
Several different C/C composites were prepared at different heat-treatment temperatures, either with or without illite addition. As the heat-treatment temperature was increased, the SiC formation via carbothermal reduction increased until all the added illite was consumed in the case of the samples heat-treated at 2,300℃ as a result of the intimate contact between the SiO2 in the illite and the phenol resin in the precursor or carbon fibers in the preform. The illite-containing composites prepared at all heat-treatment temperatures exhibited fewer pores, voids, and interfacial cracks than the corresponding composites without illite, resulting in larger bulk densities and lower porosities. A delay of oxidation was not observed in the illite-containing composites prepared at 2,300℃, suggesting that the illite absorbed energy for exfoliation or other physical changes. Thus, illite may have potential applications as a filler for C/C composites despite the low densities of the prepared composites obtained here, even at a high heat-treatment temperature of 2,300℃; however, the thermal oxidation resistance of the resulting composites was improved. Furthermore, if illite-containing C/C composites with densities comparable to those of typical C/C composites can be realized in future studies, the resulting composites could readily be used in practical applications.