Removing harmful volatile organic compounds (VOCs) has been particularly important in environmental science and technology. In particular, benzene, toluene, and xylene, referred to as BTX, are notorious for causing human disease; therefore, much effort has been made to remove these compounds efficiently from indoor air and water[1-5].
Many different methods can be used for removing VOCs. Photocatalysis can be used to decompose VOCs to water and CO2. In photocatalysis, a semiconductor which can absorb light produces electron-hole pairs, and the electron and hole can react with oxygen and water, respectively, to form highly oxidizing species such as O2- and OH radicals. These radicals can decompose organic pollutants to CO2 and water. Most photocatalysts can absorb only UV light due to a large band gap; therefore, photocatalytic activity under sunlight is very low. Much effort has been devoted to reducing the band gap of the photocatalysts so that visible light can also be absorbed. Another possibility for removing VOCs is the use of highly efficient adsorbents which can remove pollutants even under dark conditions. In principle,adsorbents cannot be used more if all adsorption sites are occupied; however, by using various methods, adsorbents can be regenerated and reused [6-10].
Activated carbon, due to its large surface area and high porosity, has been widely used as an adsorbent of VOCs [6,7,9,10]. Some improvement of the adsorption capacity of activated carbon has been achieved by using surface modification techniques. Recently, other carbon structures also have attracted attention as adsorbents of VOCs [7]. It is important to mention that most of the adsorption experiments to date have been performed under atmospheric pressure conditions. To determine adsorption behaviors more precisely, adsorption experiments under high-vacuum conditions are required.
Adsorption of VOC on adsorbents has been widely studied under atmospheric pressure conditions. To shed light on the adsorption behavior of VOCs on a surface without influence of any atmospheric impurities, adsorption studies under high-vacuum conditions are needed. In the present work, we show a novel experimental set-up, which allow measurement of the adsorption capacity of a VOC under high-vacuum conditions. Using this set-up, adsorption of toluene on activated carbon, multi-walled carbon nanotubes (MWCNTs) and nanodiamonds was measured. We found that MWCNTs and nanodiamonds show a higher capacity to adsorb toluene than activated carbon with a high porosity, indicating that the existence of pores does not necessarily result in enhanced toluene adsorption capacity. MWNTs and nanodiamonds show similar toluene adsorption capacities, implying that the carbon surface structure is not critical in determining toluene adsorption capacity.
An MWCNT material was purchased from Hanhwa Nanotech (Incheon, Korea), a nanodiamond material was purchased from Mikrodiamant (Lengwil, Switzerland), and activated carbon was purchased from Aldrich (Saint Louis, Missouri, USA). To analyze the structures of these carbon-based materials, X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FT-IR) were used. For the XPS measurements, an ultrahigh vacuum system equipped with a concentric hemispherical analyzer was used. An MgKα X-ray source was used for the XPS measurement. Transmission electron microscopy (TEM) was also used to study the geometric structures of carbon-based materials. The surface area of each sample was determined using the Brunauer-Emmet-Teller (BET) method.
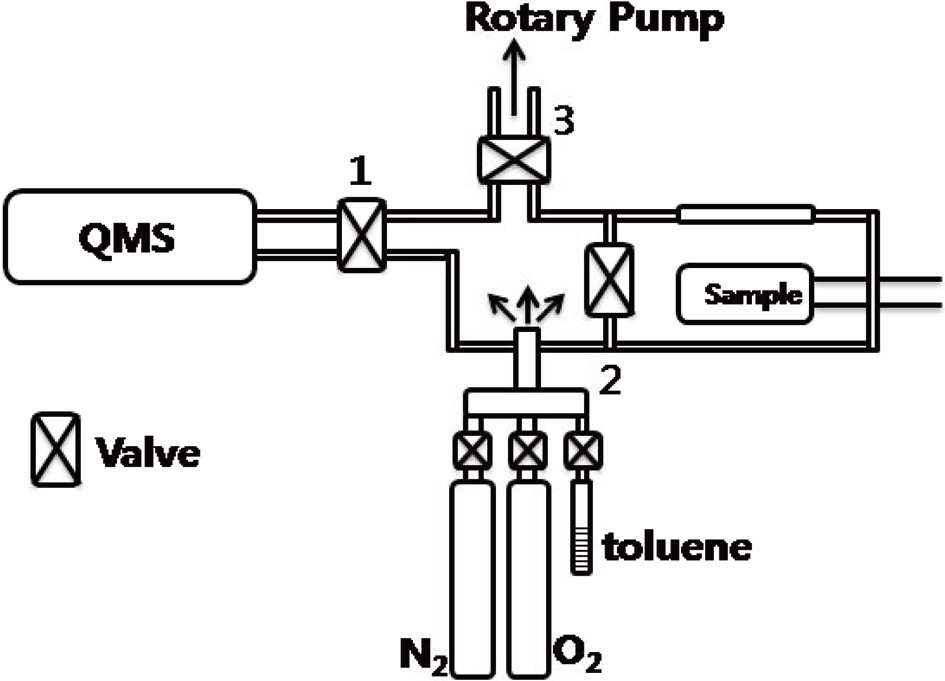
Fig. 1 shows a schematic description of the experimental setup used to determine the adsorption capacity of activated carbon,the MWCNTs and the nanodiamonds. First, a high-vacuum chamber with a sample was evacuated by a rotary pump and a turbo pump. Then, valves 1, 2, and 3 were all closed. A mixture of N2 (3 torr) and toluene (1 torr) was introduced into the chamber, and the partial pressure of both gases could be measured using a vacuum gauge. Valve 2 was subsequently open so that the sample could be exposed to the gas mixture. N2 is inert, so no adsorption of N2 took place on the sample surface at room temperature; however, toluene can eventually adsorb on the surface. When toluene adsorption takes place, the ratio of N2 and toluene changes. This can be measured by a quadrupole
mass spectrometer. A quadrupole mass spectrometer is installed in a high-vacuum chamber, and there is a leak valve between the reactor and the high-vacuum chamber. A small amount of gas from the reactor can be leaked into the high-vacuum chamber with a mass spectrometer, in which the relative partial pressures of the gas mixture can be determined. Since the volume of the reactor and the initial partial pressure of toluene are known, the number of toluene molecules adsorbed on the surface can be determined. Generally, temperature-programmed desorption (TPD) can be used to determine the adsorption capacity of the surface; however, a quantitative analysis of the absolute number of adsorbed molecule is difficult using TPD. In our experimental set-up, in contrast, determination of the absolute number of adsorbed molecules can be made. Samples were pelletized for toluene adsorption experiments.
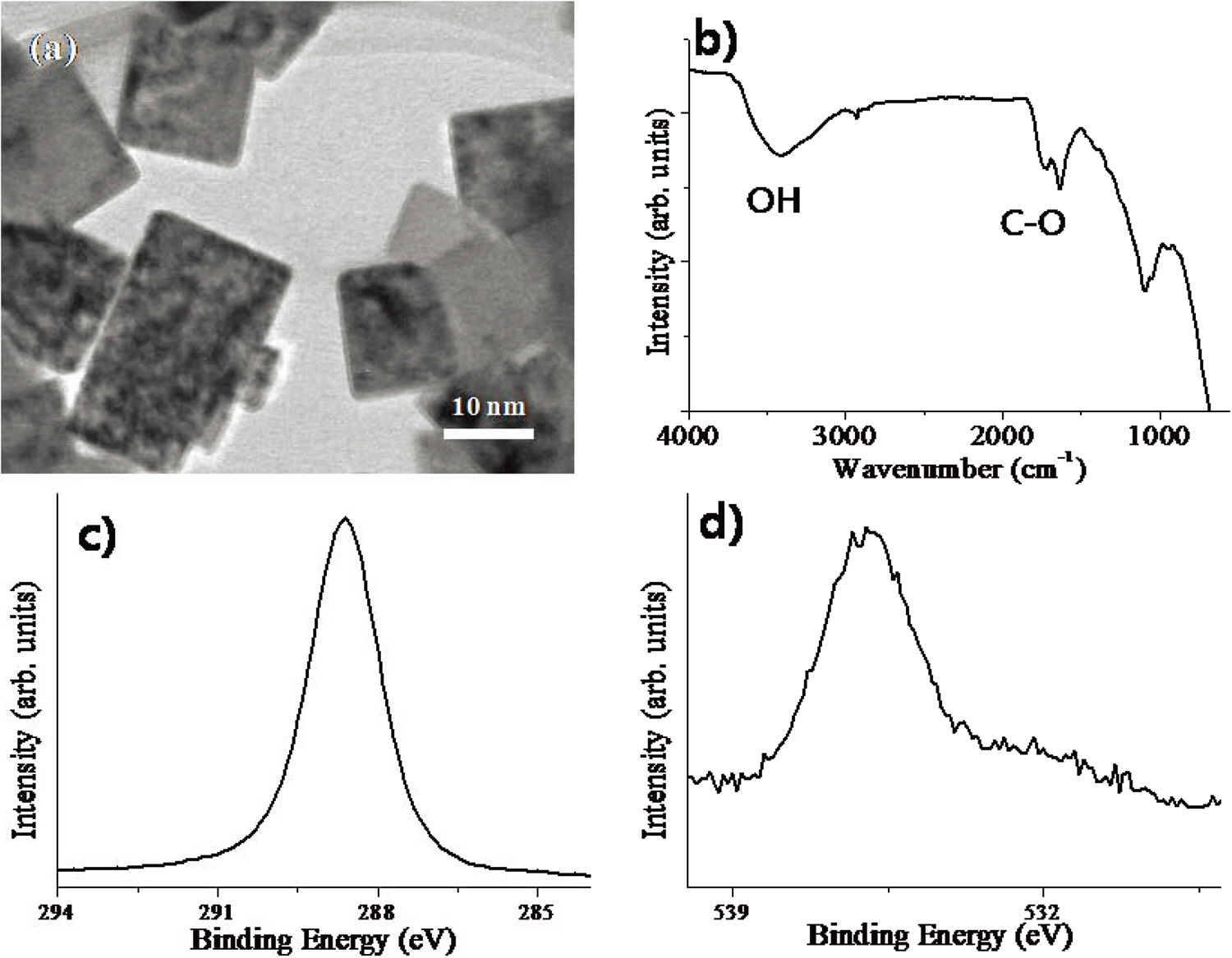
Fig. 2 shows the results of the characterization of the nanodiamonds used for the toluene adsorption experiment in this work. Using TEM, a mean particle size of about 10 to 30 nm can be identified. The surface structure of the nanodiamonds is questionable since sp3 carbon should form dangling bond on the surface, which is very unstable. To examine the surface structure of nanodiamonds, IR spectrum data was collected. Many functional groups can be identified, indicating that the surface carbon atoms of nanodiamonds should be partially oxidized (Fig. 2b). In XPS spectra, also, a large O1s spectrum intensity can be identified, which is in agreement with the IR spectrum (Fig. 2c and d). Using BET, the specific surface area of the nanodiamonds was determined to be ~80 m2/g.
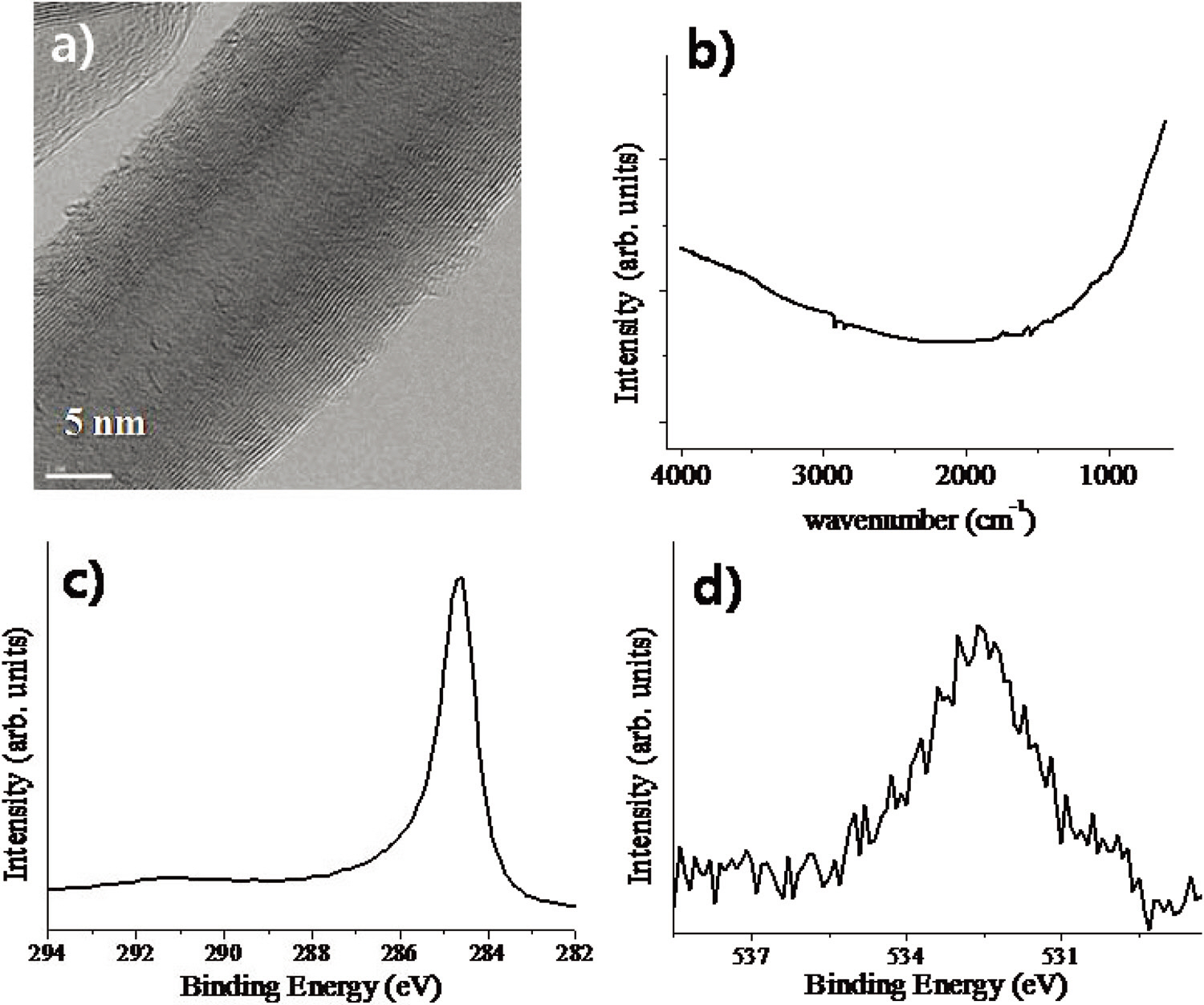
Fig. 3 shows characterization data of the MWCNTs used in this work. The TEM image clearly shows the multi-walled structure of a carbon nanotube. The diameter of the internal wall was found to be less than about 10 nm. The carbon nanotube surfaces consist of very stable sp2 carbon atoms, and no functional groups could be found in the IR spectrum. In XPS, also, the peak in the O1s spectrum was much less pronounced than in the case of nanodiamonds. In the C1s spectrum, a broad feature at about 290 eV corresponding to the π-π* excitation can be identified, which is unique for the sp2 network of carbon atoms. The BET surface area was found to be about 230 m2/g.
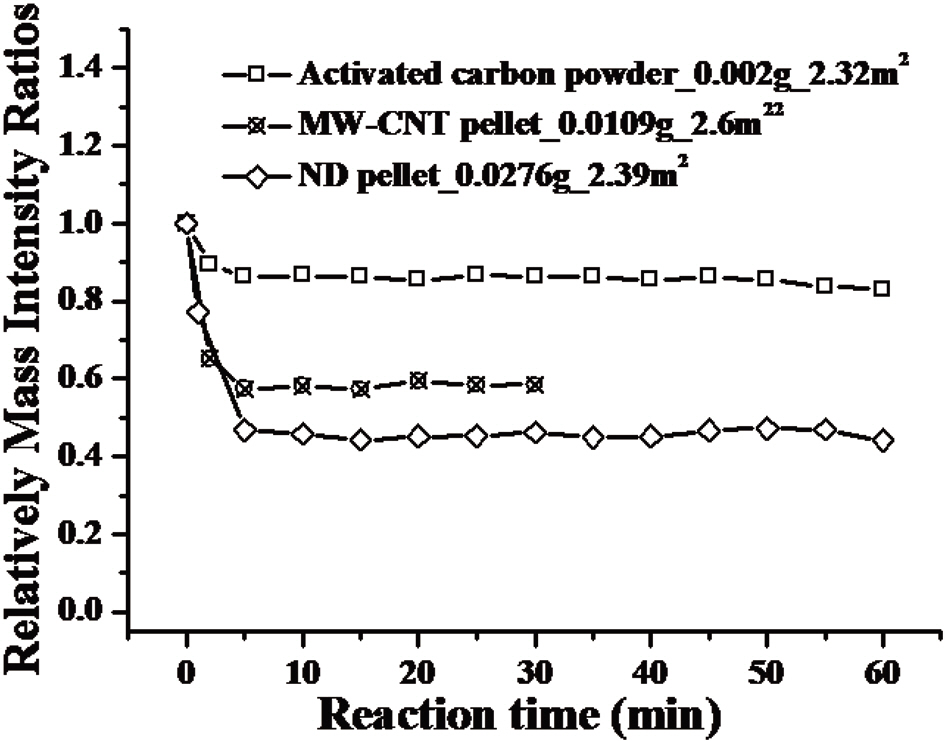
Fig. 4 compares the toluene adsorption capacities of activated carbon, carbon nanotubes, and nanodiamonds. The surface area of activated carbon (1,100 m2/g) was much higher than those of the other samples. We adjusted the weight of each sample so that the weights of all three samples became similar. As seen in the drop of the relative partial pressure of toluene as a function of time in Fig. 4, MWCNTs and nanodiamonds show higher toluene adsorption capacities than that of activated carbon. It is well known that activated carbon contains pores with a mean diameter of less than a few nanometers. This result shows that the nonporous structures of MWCNTs and naondiamonds are more reactive for toluene adsorption than porous structures. It is remarkable that the surface structure (or functionality) of carbon is not a critical factor in determining the toluene adsorption capacity since nanodiamonds and MWCNTs with completely different surface structures showed similar toluene adsorption capacities. In our novel experimental set-up, we can also
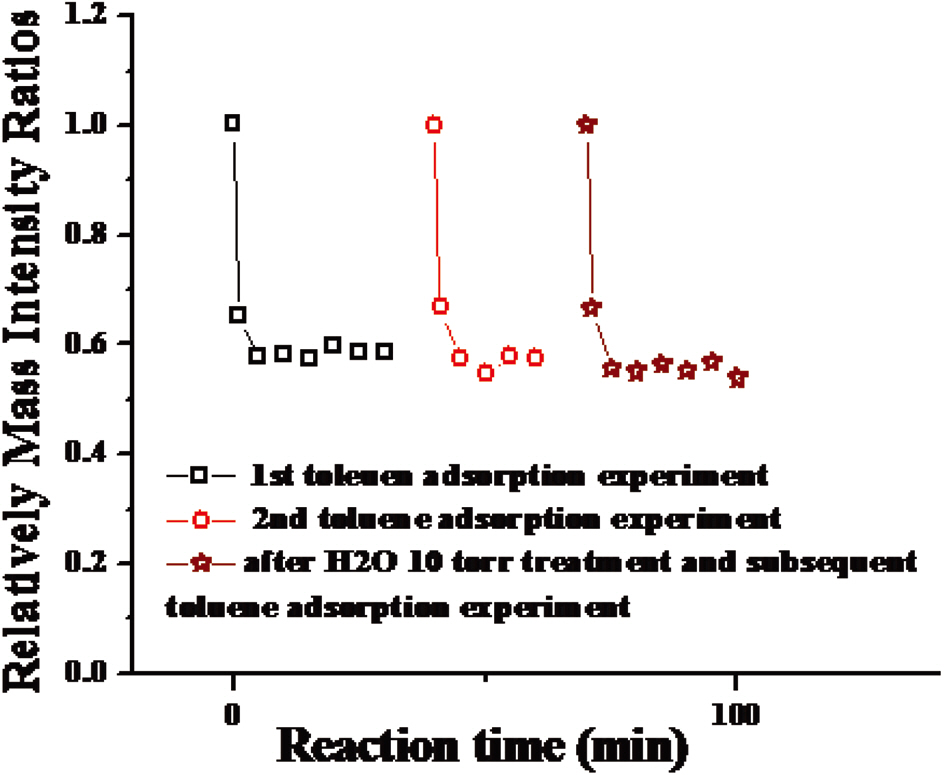
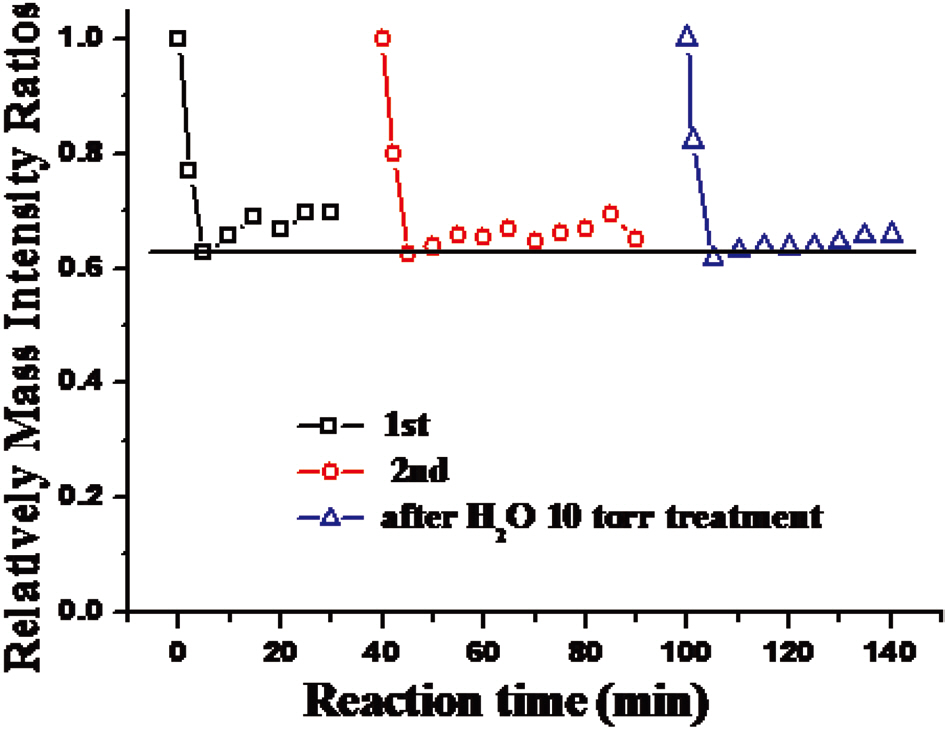
determine whether the adsorption of toluene at a given temperature is reversible or irrerversible. As seen in Figs. 5 and 6, repeated toluene adsorption experiments and evacuation of the reaction chamber resulted in the same amount of toluene adsorption. This result indicates that the adsorption of toluene is reversible since, by the evacuation process, all adsorption sites became empty so that the same amount of toluene could be adsorbed in the subsequent experiment.Even after water treatment, the same amount of toluene could be adsorbed, implying that humidity does not change the nature of the adsorption sites of the carbon nanostructures.
In summary, under high-vacuum conditions, the toluene adsorption capacities of MWCNTs, nanodiamonds and activated carbon were studied. We found that MWCNTs and nanodiamonds have higher toluene adsorption capacities than activated carbon.