Hydrogen has attracted a great deal of attention as a clean energy source and alternative fuel. Economical and efficient on-board hydrogen storage systems are critical to the success of the proton exchange membrane fuel cell technology for transportation applications [1-3]. The currently available technologies for hydrogen storage are gas compression, cryogenic liquefaction, intercalation in host metal, metal hydrides, and hydrogen physisorption. Many studies are focusing on improving present technologies and searching for advanced materials such as adsorbents [4]. Among them, carbonaceous materials are being investigated as potential hydrogen storage media because of their high specific surface area and large pore volume [5], and these carbonaceous materials include carbon nanotubes (CNTs) [6], carbon nanofibers (CNFs) [7,8], graphene [9,10], and traditional activated carbons (ACs) [11,12]. In particular, ACs have the advantage of low mass density, availability, and low cost compared with other carbonaceous materials such as CNTs, CNFs, and graphene [3,13,14]. Most of all, ACs have been widely used as adsorbent to remove organic or inorganic pollutants because of their extended specific surface area, high adsorption amount and rate, and specific surface reactivity [15,16].
Surface complexes onto carbonaceous materials are considered as an important factor in hydrogen adsorption [17]. It has been reported that hydrogen adsorption capacity on CNTs modified by acidic or basic chemical treatments are increased or diminished because of electron-withdrawing or -donating effects, and, as such, are primarily divided into acidic and basic groups, respectively [18].
In this study, we have investigated chemically treated ACs with H3PO4 concentrations. We have also studied the effects of pore structure and oxygen-functional groups of ACs on hydrogen adsorption behaviors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
2.1 Materials and sample preparation
ACs were purchased from Tokyo Chemical Industry Co.Ltd. (Tokyo, Japan) Prior to the phosphoric acid treatment, ACs were washed several times with distilled water and dried in a vacuum oven at 90℃ for 24 h. One gram of the ACs was immersed with 50 mL different concentration phosphoric acid solutions for 12 h at 40℃. The H3PO4 treated ACs samples were filtered and washed with distilled water and dried in a vacuum oven for 24 h at 80℃. The H3PO4-treated ACs had as-received H3PO4 concentrations denoted as AC-0.5, AC-1, and AC-2, respectively.
The surface analysis of ACs was studied by X-ray photoelectron spectroscopy (XPS). XPS measurements were made with a Thermo Scientific (USA), K-Alpha device with a monochromated Al Kα X-ray source (1486.6 eV). Survey spectra were recorded at O1s and C1s photoelectron peaks. The porous texture of AC was investigated by N2/77 K adsorption/desorption isotherms using a gas adsorption analyzer with a BELSORP (Japan).
The hydrogen uptake experiment was conducted under an ambient condition of 298 K and 100 bar, conditions compatible with future electric-vehicle applications. In each experiment,about 0.2 g of samples were loaded in a stainless chamber. Prior to measurement, the chamber was evacuated at 423 K for 2 h.After the chamber was cooled to room temperature, hydrogen was introduced until a pressure of 100 bar was attained. An ultra-high-purity grade (99.9999%) of hydrogen was used in this study so that the influences of moisture and other impurities could be excluded.
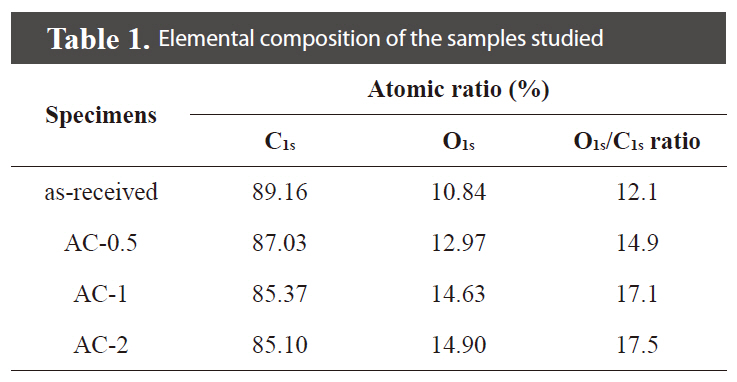
XPS analysis was carried out to analyze the elemental composition of the chemically treated ACs with the H3PO4 concentrations. The results are summarized in Table 1. After the H3PO4 treatments, the oxygen content is increased in the range between 12.1 and 17.5%. The O1s/C1s ratio is also increased with an increase in the H3PO4 concentration.
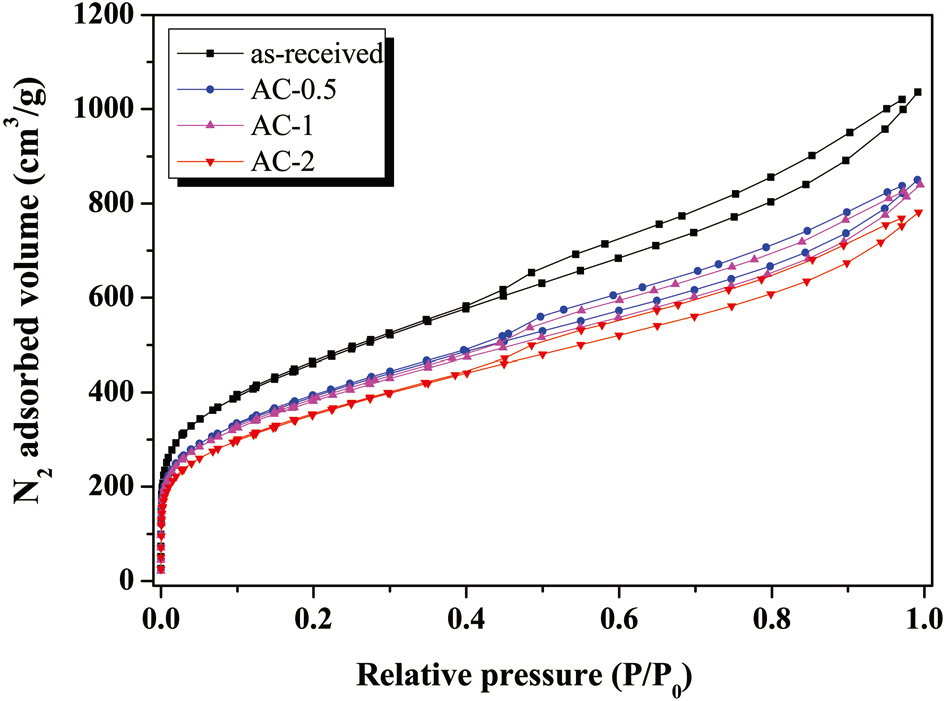
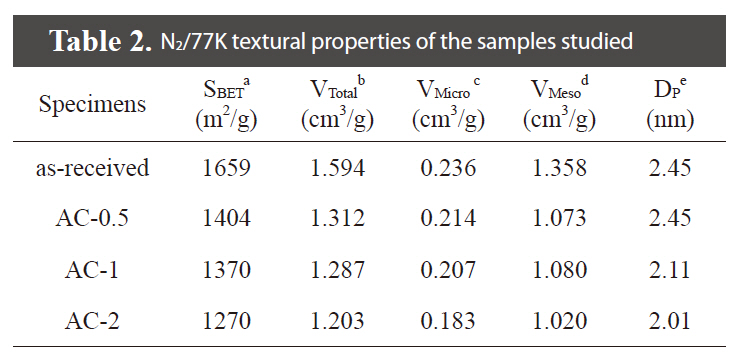
Fig. 1 shows the nitrogen adsorption/desorption isotherms for the samples studied. It was found that the all samples are composed of micropores and mesopores, showing from the Type I (relative pressure < 0.05) and Type IV (relative pressure range between 0.5 the 0.99), based on the classification recommended by International Union of Pure and Applied Chemistry [19]. Detailed information on the textural properties of the samples is listed in Table 2, and mesopore volume was determined from the Barret-Joyner-Halenda equation. As shown in Table 2, specific surface area and mesopore volumes gradually decreased with an increase in the H3PO4 concentrations. The porosities of AC-
[Table 1.] Elemental composition of the samples studied
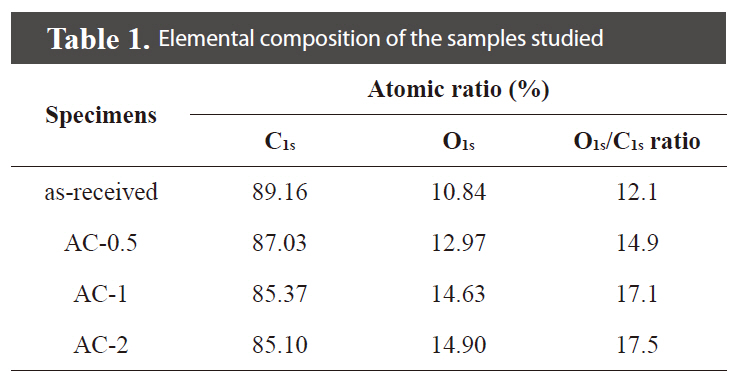
Elemental composition of the samples studied
[Table 2.] N2/77K textural properties of the samples studied
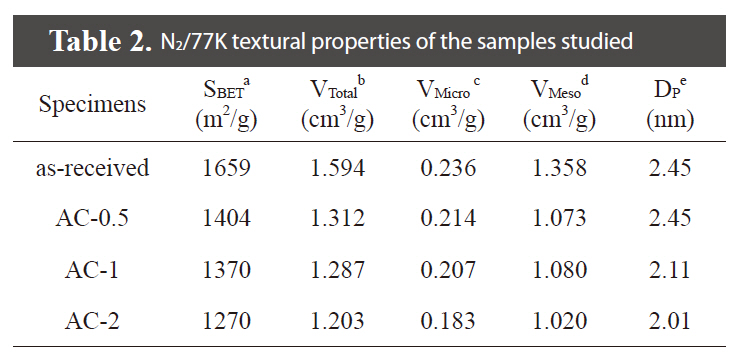
N2/77K textural properties of the samples studied
0.5, AC-1, and AC-2 were found to be 1,404 m2/g, 1,370 m2/g, and 1,270 m2/g for a specific surface area, respectively. It was found that the specific surface area and mesopore volume of all of the treated samples are slightly decreased by the introduction of oxygen-functional groups in the inner walls of pores, resulting in a decrease of mesopore sizes.
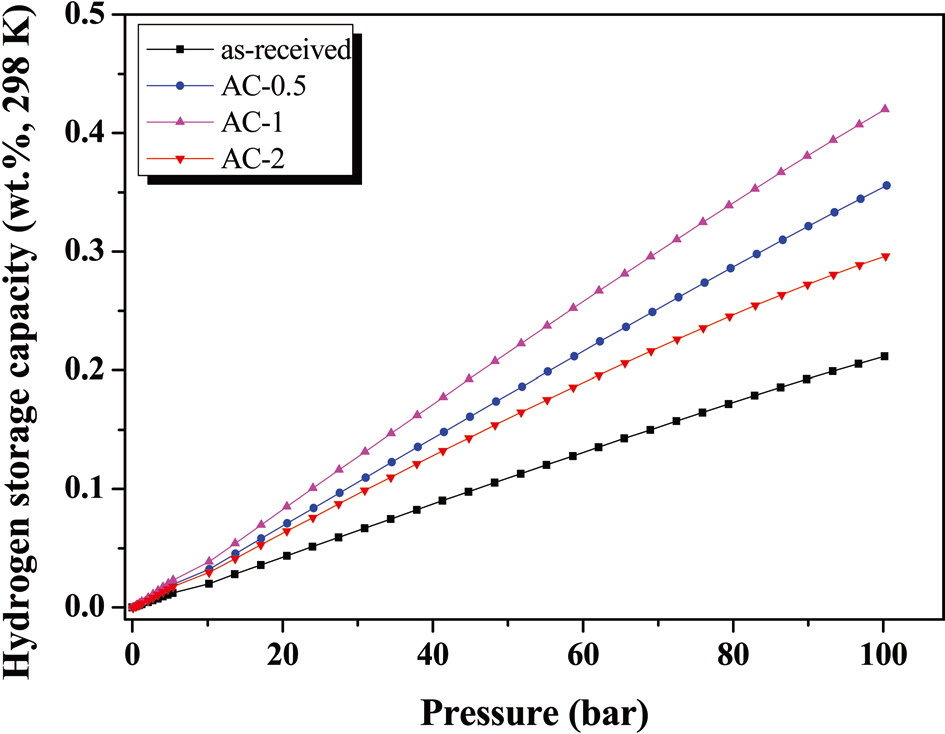
Fig. 2 shows the hydrogen adsorption behaviors of the sam-
ples.It was found that all of the chemically treated ACs showed higher hydrogen uptake than did the as-received sample. This result clearly indicates that the presence of oxygen-functional groups influences the hydrogen uptake of AC, meaning that the oxygen-functional group plays the role of more accessible sites on the ACs to hydrogen molecules. Thus, the hydrogen adsorption capacity of as-received and AC-0.5 and AC-1 samples after chemical treatments increased from 0.22 wt% to 0.37 and 0.43 wt%, respectively. From the results, the formation of hydrogenfriendly sites on AC by the H3PO4 treatment is important factor in hydrogen adsorption, resulting in electron acceptor-donor interaction at the interfaces [20,21]. It is revealed that the concentration of H3PO4 is important in enhancing the hydrogen adsorption behaviors. In addition, it is interesting note to that the hydrogen adsorption capacity of the AC-2 sample decreased to 0.30 wt%, which seems to be approaching its upper limit under these conditions.
In this work, the influence of chemical treatments with the H3PO4 concentrations on AC was investigated for hydrogen adsorption behaviors at 298 K and 100 bar. The hydrogen adsorption capacity was enhanced in the presence of oxygen functional groups for the all chemically treated samples. The chemical treatments can generate a higher amount of oxygen-functional groups and enhanced hydrogen adsorption capacity of ACs. However, in the case of excessively treated AC (AC-2), the hydrogen adsorption capacity begins to decrease. Consequently,the optimization of chemically treated ACs is essential for increasing the hydrogen adsorption capacity.