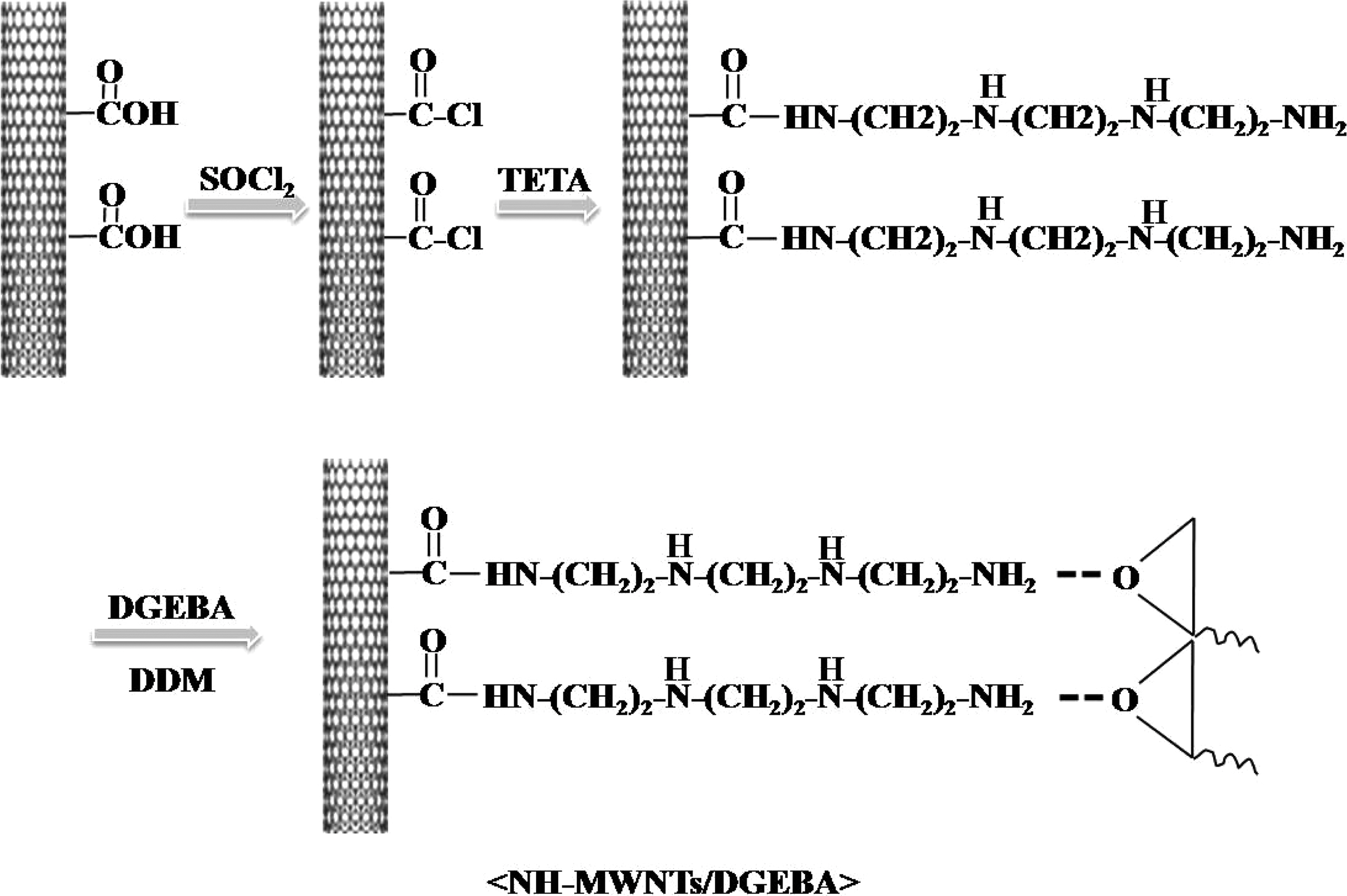
Carbon nanotubes (CNTs), which are identified by Ijima in 1991, have much attention as materials of great application potential because of their excellent chemical stability, electrical conductivity, and thermal and mechanical properties [1-3]. Among many applications, CNTs have been studied as filler for polymer nanocompoaites, such as polypropylene (PP), polyethylene (PE), poly (methyl methacrylate) (PMMA), polyamide, to improve thermal, mechanical, electrical, and rheological properties [4-7].
However, CNTs-reinforced polymer composites have following main problems: 1) the lack of interfacial adhesion between CNTs and polymer due to smooth non-reactive surface of CNTs, resulting in the decrease of load transfer from the matrix to CNTs across the CNTs/polymer interface, 2) the poor dispersion of CNTs in the polymer because of the high aspect ratio and surface energy of CNTs, leading to the aggregation between CNTs by their intrinsic van der Waals forces. Thus, these problems of CNTs may become a defect and cause the electrical and physical properties of the composite to deteriorate [8-10].
Therefore, several approaches have been developed to improve the dispersion of individual CNTs in the polymer,including in-situ polymerization, surface modification, ultrasonic treatment, etc. Among these, surface modification of the CNTs is one efficient approach to increase their dispersion and compatibility with polymer [11,12].
For surface modification, chemically covalent treatment methods have been developed to modify CNTs. The chemical functionalization is the process in which functional groups are covalently linked to the CNTs’ surface and the linkage is permanent and mechanically stable. Therefore, chemical treatment is most effective for grafting different functional groups, such as carboxyl, amine, and hydroxyl functional groups. Also, grafting amine groups onto the CNTs via chemical treatment is one of the surface modifications of CNTs because of high activity of amine groups for other functional groups. This method can lead to the enhanced dispersion of CNTs and the increase of the physicochemical properties through the reaction with polymer matrix containing polar groups or active sites [13-15].
In the present study, epoxy resin, which is the common thermosetting resin used in various applications because of their high mechanical properties, low shrinkage, good chemical and corrosion resistance, and dimensional stability, is used as the polymer matrix [16]. Multi-walled carbon nanotubes (MWNTs) were amino-functionalized using triethylenetetramine (TETA). Amino functionalized MWNTs (NH-MWNTs)/epoxy nanocomposites were prepared by melt mixing/curing process. The effect of the NH-MWNTs on mechanical interfacial properties of epoxy nanocomposites is discussed.
Multi-walled carbon nanotubes (MWNTs) produced by the
chemical vapor deposition (CVD) process were obtained from Nanosolution Co. (Korea). The properties of the MWNTs were as follows: purity>95% (C : 96%), diameter 10~25 nm and length 25~50 ㎛. Diglycidylether of bisphenol-A (DGEBA, YD-128) as epoxy resins was supplied from Kukdo Chemical Co. (Korea). The epoxide equivalent weight and a density of DGEBA were 185~190 g/eq and 1.16 g/cm3 at 25℃. The curing agent, 4,4’-diaminodiphenyl methane (DDM), and triethylenetetramine (TETA) was obtained from TCI and Aldrich, respectively.
2.2. Preparation of amine treated MWNTs
For the surface treatment, the MWNTs were acid-reacted with a sulfuric acid and nitric acid (3:1) mixture that was stirred for 6 h at 80℃. They were then washed and dried at 80℃. In the next step, the acid-treated MWNTs (A-MWNTs) were put in an excess amount of thionyl chloride and the mixture was stirred at 70℃ for 24 h. The excess thionyl chloride was decanted and the acyl-derivated MWNTs (MWNTs-COCl) were washed by THF and dried under a vacuum at room temperature. Finally, the MWNTs-COCl was reacted with excess TETA at 80℃ for 12 h and then washed by ethanol and dried at 80℃. It was named NH-MWNTs.
2.3. Preparation of NH-MWNTs/epoxy nanocomposites
The epoxy nanocomposites were prepared by a melt mixing method. DGEBA and ESO were mixed at a weight ratio of 100:0-99:1 for 1 h with sonication and were then mixed for 3 h while stirring at 80℃. The stoichiometric amount of curing agent was added to the NH-MWNTs/epoxy mixtures. The mixtures were poured into a mold and cured at 120℃ for 1 h and at 150℃ for 2 h. Finally, they were then postcured at 180℃ for 1 h in a convection oven. The preparation procedure of the NH-MWNTs and NHMWNTs/epoxy nanocomposites was presented in Fig. 1.
Structural characterization of pristine MWNTs and NHMWNTs was determined using an Fourier transform infrared spectrophotometer (FT-IR 4200, Jasco) and FT-Raman (RFS 100S, Bruker). The morphologies of the NH-MWNTs/epoxy nanocomposites were observed by scanning electron microscopy (SEM, S-4200, Hitachi). Impact-strength tests (5 mm×12.7 mm×63.5 mm) were performed using an Izod impact tester according to ASTM D 256. Their flexural properties (2 mm× 25 mm×50 mm) were measured using three-point flexural tests according to ASTMD-790 and critical stress intensity factor (KIC) of the nanocomposites were measured using a single-edge-notched (SEN) specimen in a three-point flexural test, which was conducted on an universal test machine (UTM, LLOYD, LR5K) according to the ASTM E-399. The sample size was 5 mm×10 mm×50 mm and the cross-head speed was 0.85 mm/min.
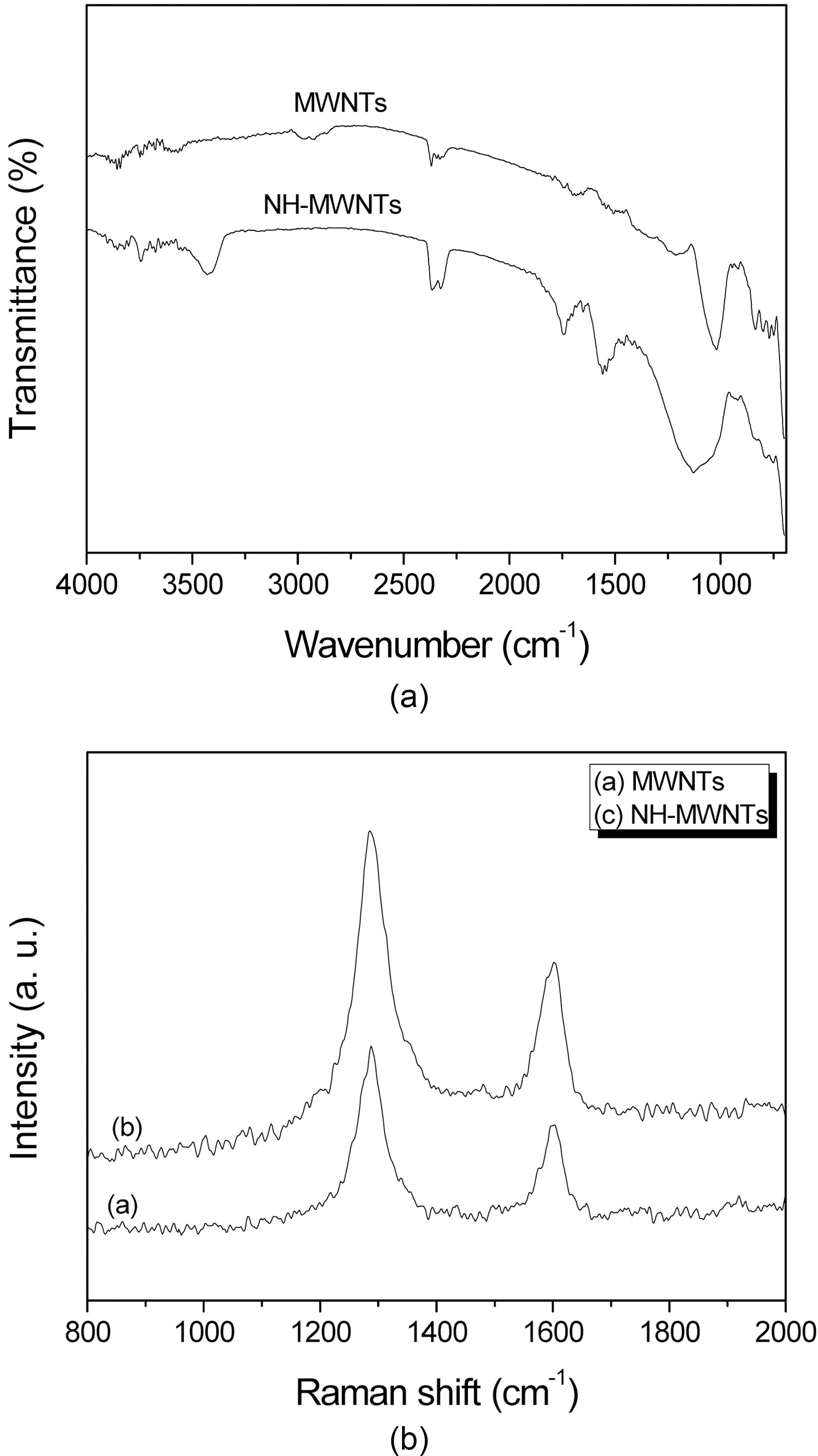
Fig. 2 shows the FT-IR and Raman spectra of pristine MWNTs and NH-MWNTs. Fig. 2 (a) shows the FT-IR spectra of pristine MWNTs and NH-MWNTs. There are no characteristics of pristine MWNTs. In NH-MWNTs, the appearance of a weak and broad band at 3420 cm-1, carbonyl stretching at 1745 cm-1, and absorbance at 1560 cm-1 is respectively attributed to hydroxyl groups, carbonyl groups, and amino groups on the surface of the NH-MWNTs, indicating the surface functionalization of the MWNTs.
From Raman spectra, the D peak (at 1290 cm-1) and G peak (at 1590 cm-1) indicate the characteristics of the MWNTs, which are attributed to the hexagonal framework of the MWNT wall. As shown in Fig. 2 (b), the intensity
ratio (ID/IG) of the D peak and G peak is slightly changed with functionalization of the MWNTs by chemical treatment. ID/IG of the NH-MWNTs (1.93) is higher than that of pristine MWNTs (1.76). This indicates that the acid treatment, acylation, and aminization of the MWNTs lead to the formation of sp3 hybridized carbon defect sites [17].
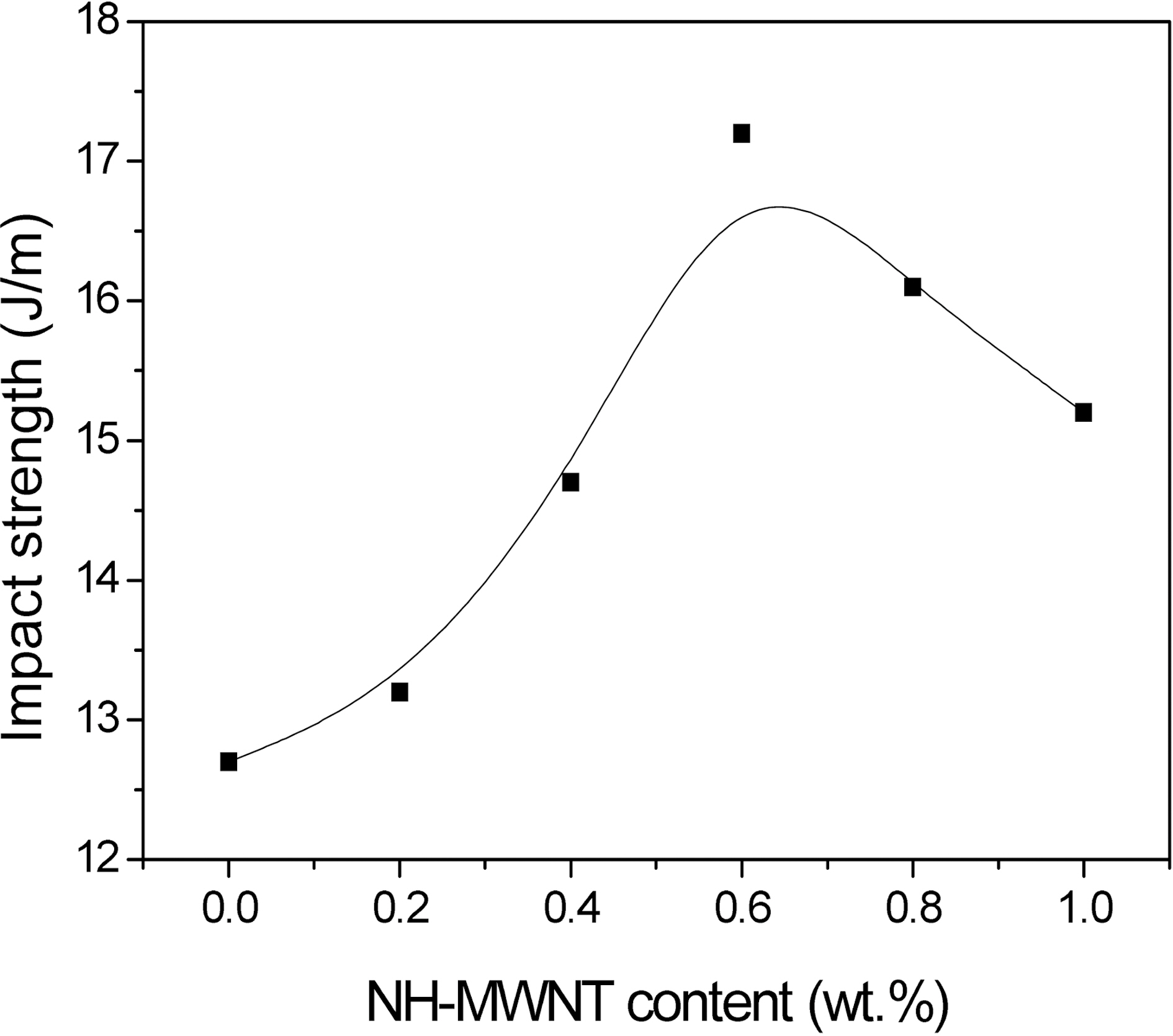
The mechanical interfacial properties of the NH-MWNTs/epoxy nanocomposites were measured through the impact strength. The impact strength of the epoxy nanocomposites with different NH-MWNT contents is shown in Fig. 3. This figure shows that the impact strength increases with increasing the NH-MWNT content. The neat epoxy is brittle, exhibiting an impact strength value of 12.7 J/m, whereas the nanocomposites containing NH-MWNTs are about 35%higher, at 17.2 J/m with 0.6 wt.% NH-MWNTs. However, a further increase in the NH-MWNT content leads to the decrease of the impact strength above 0.6 wt.% NH-MWNTs.
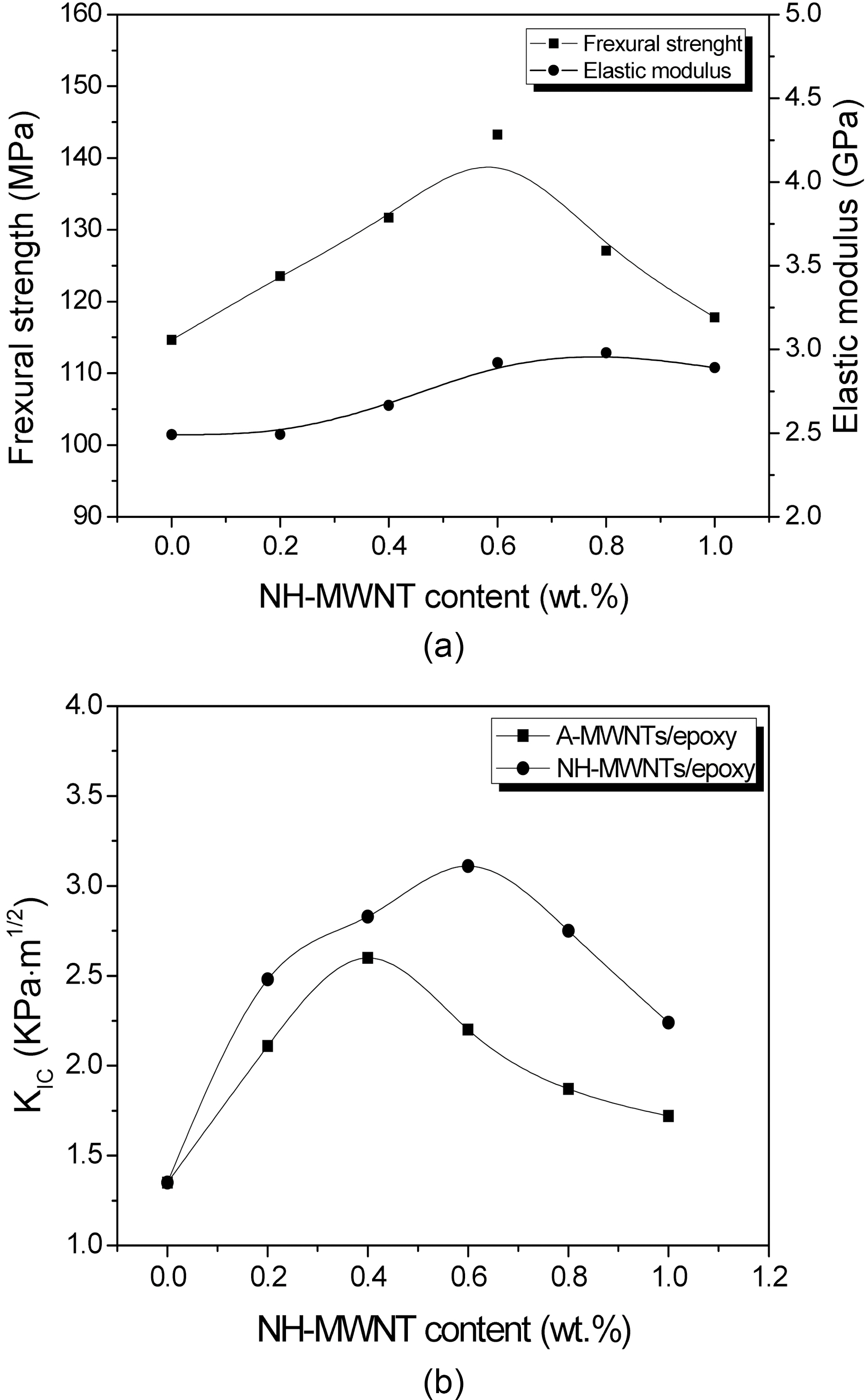
The mechanical properties of the epoxy nanocomposites are measured to determine the effect of NH-MWNT on the flexural strength (σf) and elastic modulus in flexure (Eb). The flexural strength values of the nanocomposites are determined with the three-point bending test and are calculated as follows [18]:
where
Fig. 4 (a) shows the flexural strength (σf) and elastic modulus (
To investigate the fracture toughness of the epoxy nanocomposites, the critical stress intensity factor (
here,
Fig. 4 (b) shows the
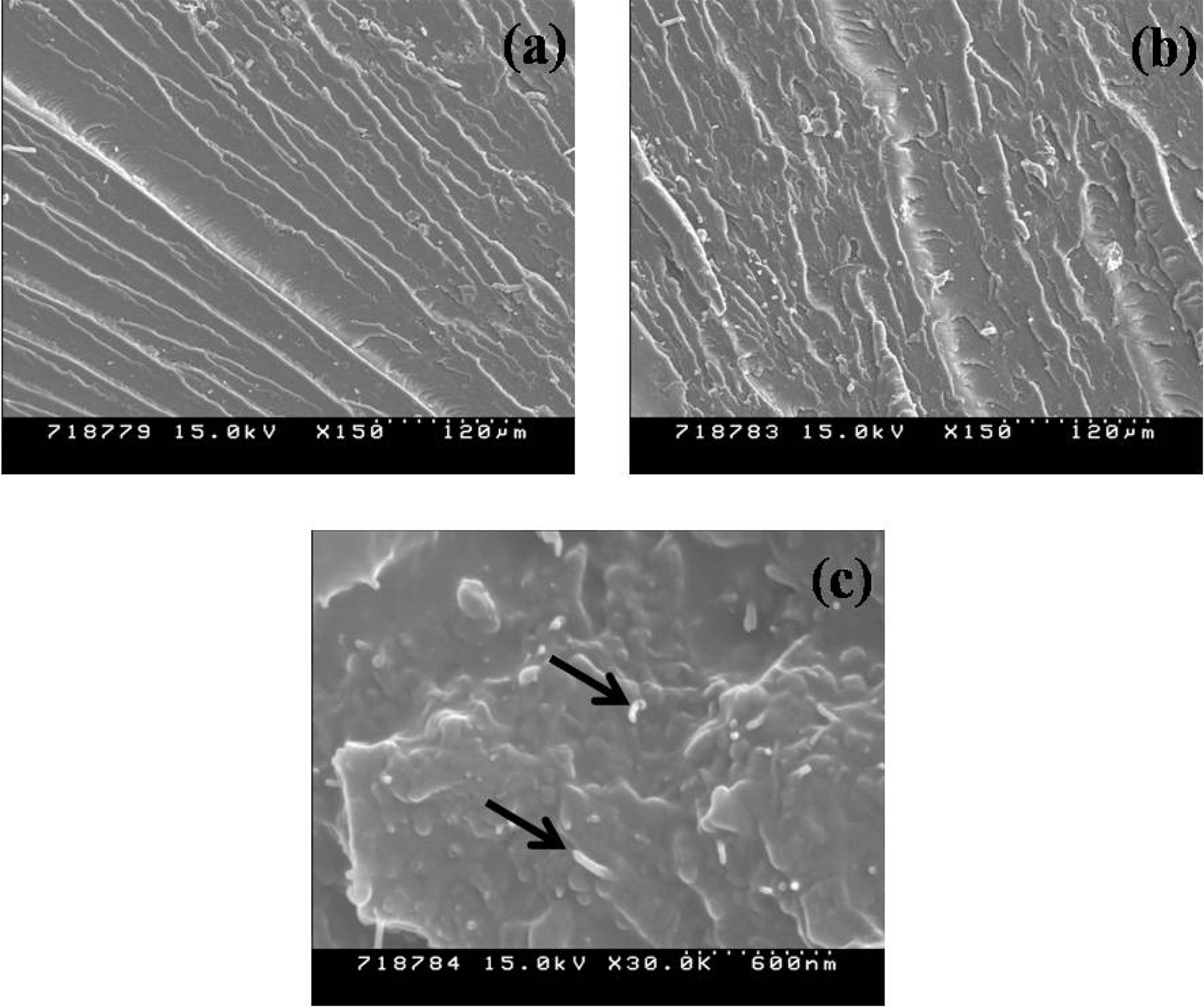
The toughness behaviors of the NH-MWNTs/epoxy nanocomposites were further characterized using SEM micrographs. The fracture surfaces of the nanocomposites after the KIC tests were examined by SEM and the results are shown in Fig. 5. From Fig. 5 (a), the neat epoxy shows one phase that is relative smooth surface, indicating a tendency to brittleness and poor mechanical properties. The surface of the NH-MWNTs/epoxy nanocomposites revealed that the NH-MWNTs are uniformly embed in and bonded with the epoxy resins, as shown in Fig. 5 (b,c). When cracks occurred in the NH-MWNTs/epoxy nanocomposites, those NHMWNTs lead to an increase in resistance to deformation and crack propagation caused by MWNTs-epoxy debonding, resulting in the absorption of more energy [21].
In this study, multi-walled carbon nanotubes (MWNTs) were chemically treated using triethylenetetramine and the effect of the amine-treated MWNTs (NH-MWNTs) on mechanical interfacial properties of the epoxy nanocomposites were investigated by means of impact strength testing, fracture toughness, and critical stress intensity factor (