



The metals and plastics used for automobiles have been painted for both decoration and protection against corrosion.The paints used contain organic polymers and solvents. The solvents contribute to air pollution, as volatile organic compounds(VOCs), when emitted during painting operations. VOCs have been the focus of environmental concern and regulated for two basic reasons: human health issues due to some VOCs being toxic and ozone formation through photochemical reactions with NOx. Recently, various efforts have been made to change the nature of paints to reduce VOC emissions [1, 2]. In order to reduce the solvent content in paints, water-based paints and powdered paints have been developed. However, water-based paints are not organic solvent free and still contain a significant amount of organic solvents along with the water. Organic solvents are also hydrophilic in nature, e.g., alcohols, ketones,and glycolethers, etc., compared to those in solvent-based paints that contain a significant portion of hydrocarbons, such as toluene and xylenes. The use of water-based and powdered paints has been limited for various reasons. Meschievitz et al. [3]described the efforts made by the U.S. Council for Automotive Research (USCAR) Low-Emission Paint Consortium to improve powdered paints as an attempt to lower VOC emissions from automotive painting operations. They indicated that the use of powder clearcoat paint presented a difficult challenge, in addition to improvements in process efficiency and smoothness of the painted surface, because the appearance of the final paint layer is critical.
In addition, the overall environmental foot prints of waterbased and powder paints may not necessarily be smaller than those of solvent-based paints. For example, Papasavva et al. [4] conducted a life-cycle analysis using three paint scenarios: 1) solventbased primer→water-based basecoat→solvent-based clearcoat; 2)powder primer→water-based basecoat→solvent-based clearcoat;and 3) powder primer→water-based basecoat→powder clearcoat.In manufacturing the materials used for the three scenarios, the last was found to be associated with the least energy, water consumption,solid waste and VOCs, but exceeded the other scenarios in particulate matter, SOx, and CO2-equivalent emissions.Therefore, it is very difficult to determine if any particular paint strategy is better than another when all life-cycle emissions are considered. Presently, it is anticipated that solvent-based paints will be widely used for the foreseeable future, which implies that VOC emissions from painting will continue, but needs to be abated. In addition, most regulated toxic pollutants released to water, land, and air from a major automotive manufacturing company were due to paint solvents [5].
Therefore, in this paper, the issues of VOC emissions and their control with respect to automotive painting are reviewed and discussed in terms of current and emerging practices.
2. Current Practices in Painting Operations and VOC Control
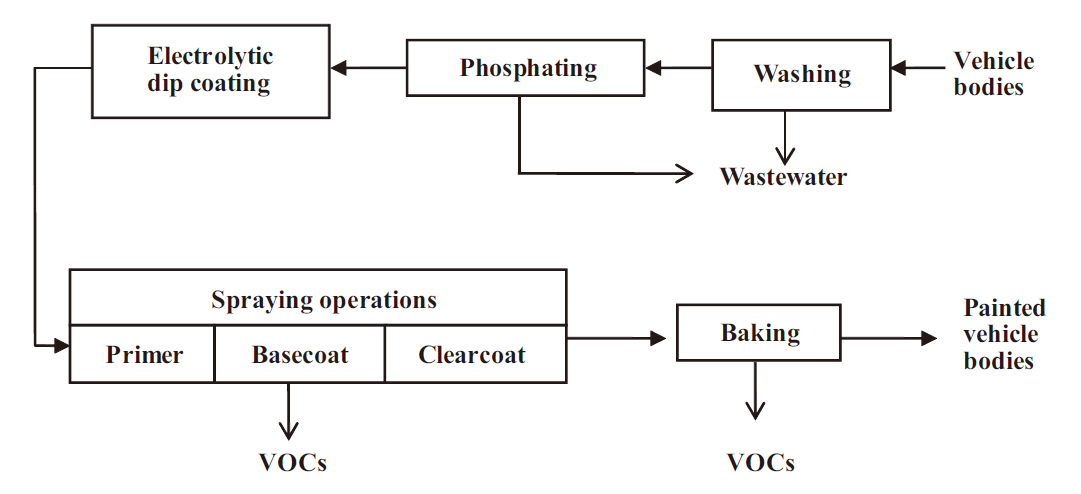
During typical painting operations (Fig. 1), metal vehicle bodies are initially put together, washed to remove the materials(including oily materials) used to protect body parts against corrosion and damage during stamping and transport, and treated with phosphating agents to prepare the surface for coating. The cleaned and surface-treated bodies are then placed in a dip tank where they are electrolytically coated with paint polymers, followed by a series of spraying operations starting with primer,basecoat, and finally clearcoat. The painted bodies are baked in an oven to cure the coated paint materials. Most VOC emissions come from the spraying operations.
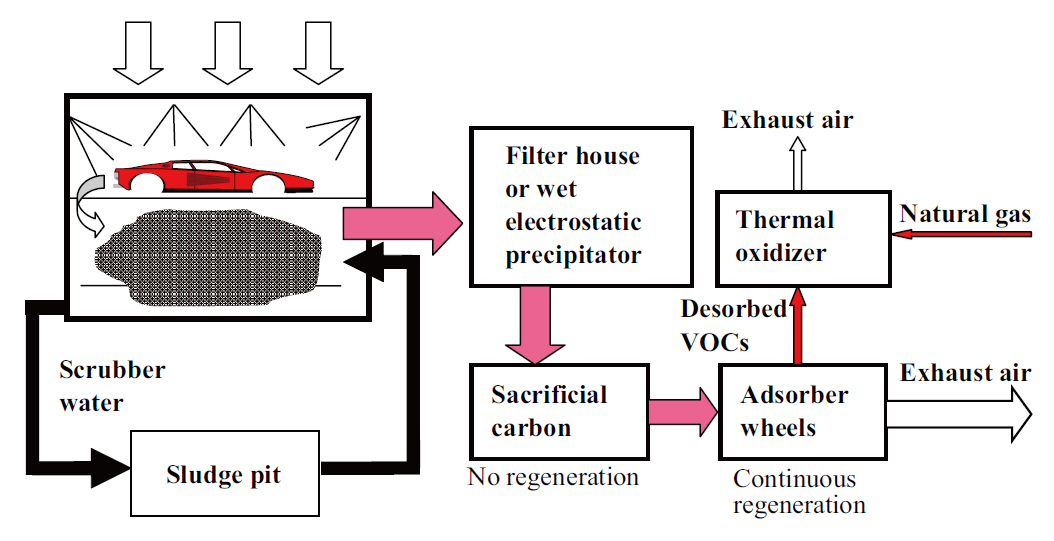
The spraying operations take place in spraybooths, which consist of a spraying section and a water scrubber system, as shown in Fig. 2. During painting, overspray paint materials are captured in the continuously recirculating scrubber water and stored in a sludge pit. The boothair that comes out of the scrubber system goes through a filter house or wet electrostatic precipitator to remove paint particulates that survived the scrubber. The VOCs in the exhaust air may have to be removed,depending on the operating permit issued by the regulatory agency, for which an adsorption process is most commonly used. The adsorption process typically consists of two sets of adsorbers: a sacrificial activated-carbon adsorber followed by a fixed-or moving-bed adsorber. By desorbing the adsorbed VOCs,a concentrated stream of VOC-laden air is produced and sent to a thermal oxidizer (RTO), where they are thermally oxidized.The second set of the adsorber is typically a moving-bed system made of rotating adsorber wheels. The exhaust air from the oxidizer,along with that from the adsorber, is emitted to the atmosphere.
As mentioned earlier, the overspray materials are captured in the scrubber water and stored in a sludge pit. These materials are tacky in nature and; therefore, have to be detackified using chemicals (called detackifiers, typically organic polymers)in the water. Depending on how the materials are detackified,the resulting materials (called paint sludge) are designed to either settle or float. When the flotation method is used, the paint sludge is continuously removed via thickening, using a flotation device, followed by dewatering via a vacuum filter coupled with a disposable fabric filter medium.
When the sedimentation method is used, the scrubber water is periodically separated from the paint sludge and discharged to a public wastewater treatment plant for treatment. The paint sludge is then removed from the pit and landfilled.
These processes are reviewed and discussed here, along with various aspects associated with improvements made to the removal of VOCs. The topics to be reviewed and discussed include:the nature of VOCs, VOC adsorption, thermal oxidation of VOCs, boothair recirculation, VOC absorption, biological VOC removal, energy recovery, and beneficial use of paint sludge.
Commonly used solvents in solvent-based paints include: aromatic hydrocarbons, aliphatic hydrocarbons, esters, ketones,alcohols, and glycolethers. Several investigators have reported on the nature of these compounds. Kim et al. [6] grouped the solvents used in solvent-based paints at an assembly plant into hydrophobic and hydrophilic types. The hydrophobic group contained aromatic hydrocarbons (e.g., toluene, xylenes, ethylbenzene,1, 2, 4-trimethylbenzene and naphthalene), aliphatic hydrocarbons (e.g., n-heptane and naphtha), and other hydrocarbon mixtures (e.g., mineral spirits). The hydrophilic group contained ketones (e.g., acetone, methyl ethyl ketone, methyl isobutyl ketone, methyl amyl ketone, and di-isobutyl ketone),esters (e.g., ethylacetate, n-butylacetate, ethyl propionate and isobutyl isobutyrate), alcohols (e.g., methanol, ethanol, propanols,and butanols) and glycolethers (e.g., butyl cellosolve acetate and butyl carbitol). The grouping was based on the relative magnitudes of the Henry's law constants of the solvents, as reported by Kim et al. [7], who showed that the constants were approximately 6,000 atm cm3/mol (610 Pa cm3/mol) for aromatic
[Table 1.] Solvent usage rates at typical Ford plants in North America [6]
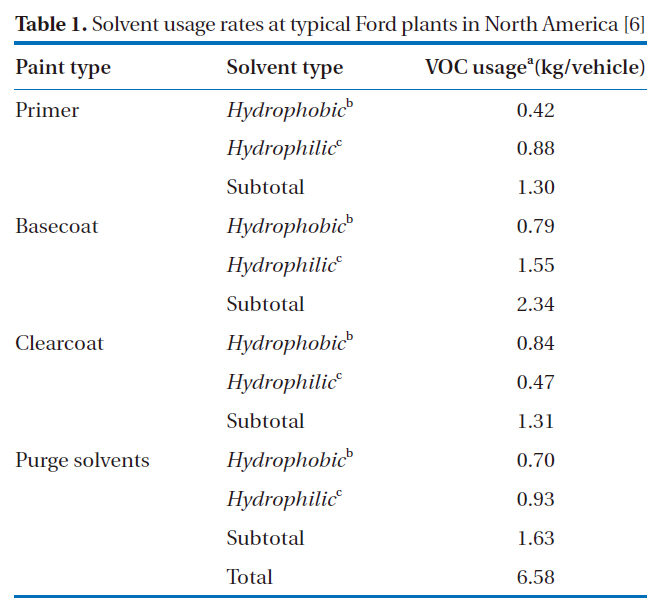
Solvent usage rates at typical Ford plants in North America [6]
hydrocarbons; 50-150 atm cm3/mol (5.1-15.2 Pa cm3/mol) for ketones; 10 atm cm3/mol (1.01 Pa cm3/mol) for alcohols; and 0.005-5 atm cm3/mol (0.00051-0.51 Pa cm3/mol) for glycolethers.Kim et al. [6] also reported typical solvent usage rates at Ford in North America at that time, which are shown in Table 1.
Basically, the same set of compounds was found in the paint solvents used in the Taiwan automotive industry [8, 9]. The compounds included toluene, xylene, ethyl acetate, n-butyl acetate and ketones. In a survey conducted by Chang et al. [9] to determine the volume and composition of the exhaust gases from 80 stacks of five assembly plants in Taiwan, they found that: 1)VOC emissions from electrolytic coating were low; 2) those from spray painting (primers and top coats) were the major sources(about 85% of the total VOC emissions); 3) about 70 to 80% of VOCs were emitted from the spraybooth system, while 10 to 20%were emitted through drying ovens; and 4) emitted VOC concentrations were about 30 ± 15 ppm from the spraybooth and about 125 ± 45 ppm from drying ovens. The reported VOC concentrations are somewhat lower than those observed in the U.S.plants, which were reported to be 50 to 200 ppm in the spraybooth exhaust air [10]. Yuan et al. [11] found that the composition of VOCs in air samples taken near an automobile factory in Beijing were 35.2% m/p xylenes; 25.7% toluene; 11.1% ethylbenzene and 6.8% o-xylene, and aromatics accounted for 96.9% of the total VOCs. Anderson et al. [12] reported that the spraybooth air from the automated clearcoat paint zones at a Ford plant using solvent-based paints primarily contained aromatic hydrocarbons,with lesser amounts of alcohols (butanol, 2-propanol and isobutanol), a glycol ether (butyl cellosolve), ketones (methylisobutyl ketone and acetone) and an ester (n-butyl acetate).
Isocyanates associated with high-solid enamel paints may be toxic to humans and; therefore, require additional control measures[13].
As mentioned earlier (Fig. 2), the VOC-laden air from the scrubber goes through a sacrificial-carbon adsorber, which is a fixed-bed system containing cartridges of pelletized activated carbon. The carbon is called “sacrificial” because it is not designed to be regenerated, but periodically replaced (a few years or longer) once it has become saturated with “heel” compounds.The sacrificial carbon is used to: 1) protect the more costly adsorber wheels that follow the trapping heel compounds, which are relatively high boiling-point, irreversibly-adsorbing VOCs, as well as particulates that escape the preceding scrubber and filter systems, and 2) moderate the VOC concentrations in the incoming boothair by shaving peaks and valleys of the fluctuating VOC concentrations caused by the intermittent nature of painting operations. In other words, VOCs are adsorbed during the peaks and desorbed during the valleys. Consequently, the VOC concentrations in the air from the sacrificial-carbon bed remain relatively constant during normal operating conditions. The VOCs at this point are relatively hydrophobic (e.g., hydrocarbons),because most hydrophilic VOCs (e.g., alcohols and glycolethers)are captured in the spraybooth scrubber water, although some are slowly released by stripping during non-painting periods.
The air from the sacrificial-carbon bed is sent to an adsorption system that contains activated carbon, zeolite, or a combination of the two [10, 14]. This adsorber can be a fixed or moving bed. The most commonly used moving-bed system in the automotive industry is a rotating-wheel system, which contains corrugated paper laden with activated-carbon fibers or zeolite.Activated carbon has been the most widely used adsorbent for the adsorption of VOCs, but zeolite has also recently been used for removing relatively polar oxygenated solvents because of its relatively polar nature [14]. The adsorber used in this system was continuously regenerated on a cyclic basis (90% of the wheel in adsorption and 10% in desorption) [10]. The regeneration occurred as the wheels rotated through a heated desorption zone at ~130℃ for the activated-carbon systems [12] and ~180℃ for the zeolite systems [12], which produced a desorbed VOC-laden air. The VOCs in the desorbed air were concentrated approximately 10 fold compared to those in the incoming air [10, 14,15]. Therefore, the use of adsorption has the benefit of reducing the size of the downstream treatment process, which is thermal oxidation in this case, approximately 10 fold. In the thermal oxidizer,the captured VOCs were thermally oxidized. The VOC removal efficiency via this adsorption system has been reported to be around 90-99% [14, 16].
One major operating issue associated with rotating-wheel adsorption systems is the accumulation of irreversibly adsorbed organics onto the adsorption medium, especially activated carbon,due to the use of a relatively low desorption temperature.The irreversibly adsorbed organics are the heel compounds that survived the sacrificial-carbon bed, which could adversely affect the adsorption of VOCs. The heel compounds may include some esteric detackifiers (to be discussed later) that may become volatilized in the scrubber.
Humidity is known to affect the adsorption of VOCs. Tao et al. [17] showed that humidity adversely affected the adsorption of three VOCs; methanol, acetone and benzene, onto zeolite 13 ×with respect to the breakthrough time and VOC removal. The effect was found to be greater with benzene than the other VOCs,due to their greater polarity compared to benzene.
Some VOCs may undergo oxidation reactions while being adsorbed.Popescu et al. [18] studied the dynamical adsorption of typical car paint solvents at 23℃, i.e., toluene, butylacetate and butanol, using two microporous, extruded activated carbons(surface areas of 1,000-1,200 m2/g), and the thermal regeneration of these carbons with hot air at 150℃ using thermogravimetry.A sequence of seven adsorption?desorption cycles, with a mixture of these solvents, left the carbons with some textural changes, but their adsorption capacities remained virtually unaffected.Temperature-programmed desorption experiments,using carbon samples heated in a helium flow containing oxygen traces, provided evidence of desorption of numerous oxidation products. However, it was not clear whether the reactions occurred during adsorption or desorption. Regardless, the oxidation reactions did not seem to affect desorption of the parent VOCs or the oxidized compounds.
The use of these rotating-wheel adsorption systems has a limitation with respect to their ability to concentrate VOCs about 10 fold due to the safety limit the VOCs in the desorbed air are allowed to reach [15]. It has been reported that about a 2,000-fold increase in VOC concentration can be achieved using a fluidized-bed activated-carbon adsorber [12, 15]. The medium used in the adsorber was attrition-resistant activated-carbon beads, with nitrogen used as the desorbing gas to overcome the low safety limit of the VOCs when air was used as the desorbing gas. Thus, desorption was carried out continuously in a separate desorbing section of the fluidized-bed adsorber, but at a higher temperature (up to 370℃) than that (~130℃) used for the rotating-wheel system [12].
Anderson et al. [12], via a 6-month pilot study, investigated the applicability of a fluidized activated-carbon adsorber to spraybooth air, where the VOCs were primarily aromatic hydrocarbons,at a Ford assembly plant, as an alternative to the rotating-wheel system. The activated carbon had a surface area of 1,100-1,300 m2/g. The results showed VOC removal efficiencies from 96 to 99%, which was basically the same as that expected from a typical rotating-wheel adsorber, with virtually complete regeneration of the spent carbon beads at the desorption temperature of ≥340℃. A 1,000-to-3,000 fold increase in VOC concentration was observed, which was close to the theoretical 2,000-fold value calculated for the tested operating conditions.Compared to a typical rotating-wheel system with an RTO, the fluidized-bed system was estimated to require 80 to 90% less energy costs and lower capital costs due to the smaller RTO needed.
Regenerative RTOs are the most commonly used type in the automotive industry [19], which has two heat-storage sections,with a ceramic heat-transfer medium for recovering and storing the heat liberated in the combustion chamber situated between the two heat-storage sections. The desorbed VOC-laden air from an adsorber enters one of the two sections, is heated via the heated medium, and sent to the combustion chamber,where the VOCs are thermally oxidized with natural gas. The hot exhaust gas from the chamber enters the other heat-storage section,where it heats the medium. Once the medium in the first heat-storage section cools to a preset design temperature, the flow of air is reversed so that the VOC-laden air enters the other section, section to become subsequently heated there this process continues. The temperature in the combustion chamber is typically >650℃ [10, 19]. The ceramic medium is typically randomly packed materials, with no catalysts.
The energy required to operate an RTO typically comes from natural gas, because the energy provided by the VOCs in the incoming air is insufficient. At Ford, the energy produced from the VOCs during painting typically accounts for about 15% of the energy demand for an RTO [10, 15]. Therefore, energy consumption is an important operating-cost issue. Some efforts have been made to reduce the energy demands by introducing catalysts into the RTO system, since most RTOs are used without a catalyst.A Ford assembly plant retrofitted an RTO to a catalytic unit,called a regenerative catalytic oxidizer (RCO) [20], using a basemetal-oxide catalyst. The reason for the switch to an RCO was to reduce the oxidation temperature from ~760 to ~420-450℃;thus, reduce the energy consumption (from 2 million Btu/hr[590 kW] to 0.55 [160 kW]), increase the capacity and also reduce the production of NOx. The retrofitting was accomplished by replacing half of a 2.1-m ceramic medium bed with 0.3 m of the catalyst. The RCO showed a VOC destruction efficiency of ~96%compared to the ~98% of the original RTO, and the activity of the catalyst was not lost after one year of operation.
Recirculation of VOC-laden spraybooth air has been used as an alternative to carbon adsorption for increasing the VOC concentration in the air and; thus, reduce the air volume requiring subsequent treatment via thermal oxidation or carbon adsorption.The recirculation can only be used in automatic spraying sections, not in manual spraying sections, because of the potential adverse health hazards associated with elevated VOC concentrations in the spraybooths of automatic sections, as well as the generally low VOC concentrations in spraybooths of the manual sections [10]. However, the test results at Ford indicated that air recirculation was less efficient than rotating-wheel adsorbers at concentrating the VOCs due to relatively large fugitive losses associated with air recirculation [10].
As mentioned earlier, the spraybooth air first passes through a water scrubber, where some of the VOCs are captured by absorption.However, the scrubber is typically designed to capture overspray paint materials, but not VOCs. Therefore, the captured VOCs are eventually stripped during non-painting periods,which is dependent on the Henry’s law constants of the VOCs.The Henry’s law constants of paint solvents typically ranges from 6,000 atm cm3/mol, for toluene, to approximately 0.003 atm cm3/mol for n-methyl-2-pyrrolidinone [7]. Hydrocarbons,such as toluene, can be stripped in a few minutes; whereas, glycolethers,such as butyl cellosolve and butyl carbitol, and aminoketones,such as n-methyl-2-pyrrolidinone, can remain with water for a sustained period of time, even with vigorous mixing.
Scrubber capturing VOCs, some inverstigators have attempted to modify the scrubber system to improve the absorption of VOCs, with their eventually removal. Mir and Zahka [21] received a patent for a process that removes VOCs from the exhaust air of paint spraybooths and paint driers by converting the water into an oil-in-water emulsion or a surfactant solution with powdered activated carbon (PAC). The concept relates to VOCs generally being considered as relatively hydrophobic and nonpolar (e.g.,hydrocarbons) in nature and; therefore, would be attracted to nonpolar materials, such as oil or PAC. Nalco developed a similar oil-in-water process for removing VOCs from exhaust air during automotive painting operations [22]. In the case of the emulsion, the spent solution was either concentrated, using ultrafiltration, or chemically broken. The resulting oily materials were then heated to strip off the captured VOCs, which were recovered by condensation. The oily materials were returned to the process for additional VOC removal. The spent PAC can be separated from water for regeneration, while the captured VOCs can be recovered.
However, the scrubbing solution in either the emulsion or the PAC slurry contains surface active agents (emulsifiers for the emulsion and surfactants for the PAC solution) that could interfere with the removal of VOCs. In the case of the emulsion,the emulsifiers would impede the mass transfer of VOCs due to their accumulation at the air/water interface. In the case of the PAC solution, the surfactants are generally strongly adsorbable and; therefore, compete with VOCs for adsorption sites. However,without a surfactant, Kim and Pingel [23] demonstrated that a PAC/water slurry reactor could provide a sink for VOCs in a spraybooth scrubber system.
The emulsion process was found to be ineffective at capturing VOCs for the reasons stated above, but was found to be effective in detackifying paint sludge in the scrubber water, as the tackiness of the sludge is reduced when mixed with the oily material.This emulsion process has now evolved into a detackification process by replacing the oil-in-water emulsion with an esteric emulsion, in which esteric compounds function as a detackifier.The esteric compounds have relatively low aqueous solubility and; therefore, need to be emulsified using either a fatty acid or a surfactant. In this detackification scheme, the paint sludge captured in the scrubber water is not separated from the bulk scrubber water using sedimentation or flotation, as in the conventional process. The sludge is completely mixed in the scrubber water and allowed to circulate through the scrubber system.In order to control the level of paint sludge accumulating in the scrubber system, a portion of the emulsion is continuously replaced with fresh emulsion. The removed emulsion is processed to remove the paint sludge, and then returned to the scrubber water system. However, there is a potential concern with the use of esteric detackifiers as they may be carried into the subsequent adsorption system and possibly irreversibly adsorb onto the carbon media in both sacrificial-carbon beds and carbon wheels,which would lower the VOC adsorption capacity and; thus, adversely affect the VOC removal efficiency of the system.
Pierucci et al. [24] developed an absorption process, in which water was replaced with an oily material. Here, the boothair and oily material flowed in a countercurrent fashion at a mass ratio of approximately 1; therefore, the VOCs in the air were absorbed into the oily material at a low temperature. The VOCs absorbed into the oil were then stripped at a high temperature in a vacuum system, and subsequently condensed at a temperature slightly below the ambient temperature. The oil, after removing VOCs by stripping, was sent back to the absorption process for absorption of the VOCs. The authors reported a recovery performance of approximately 90% at an industrial site located in Italy that treated a gas stream with a VOC content of 1,200 to 2,500 ppm,at a flow rate of 14,000 Nm3/hr, using an oil flow rate of slightly more than 10 m3/hr. This VOC concentration was more than 10 times larger than that found in typical automotive spraybooth air and; therefore, it was not clear if this process would be either technically or economically feasible with respect to automotive painting.
As mentioned earlier, a typical assembly plant has a water scrubber to partly control the overspray paint materials. Although the scrubber was not originally designed to capture VOCs, an attempt was made by Kim et al. [6, 25] to see if an existing scrubber could be converted into an aerobic biological reactor for the biological degradation of VOCs as they were captured in the scrubber water. A pilot-scale experiment was conducted at an assembly plant for over 400 days using the scrubber water supplemented with nutrients [6]. To simulate the scrubbing conditions in spraybooths, a mixture of four paint solvents (toluene, n-butanol, methyl ethyl ketone [MEK] and butyl cellosolve [BC]) was fed into the reactor via volatilization into the air supply. The volatilities and aqueous solubilities of paint solvents vary greatly. The above four solvents represent the four major paint solvent groups; hydrocarbons, alcohols, ketones and glycolethers. The Henry’s law constants of the major groups vary by over five orders of magnitude [7, 21]. Hydrocarbons(e.g., toluene) are the most hydrophobic and not expected to be satisfactorily captured; whereas, alcohols, ketones and glycolethers are relatively hydrophilic. The results obtained from the pilot experiment showed that: 1) all the biodegradable organics were removed at an organic loading rate greater than the maximum solvent capture rate at a typical assembly plant,which means that the size of the scrubber at a typical assembly plant was sufficiently large to handle the solvent loading under normal production conditions should the scrubber be converted to a bioreactor; 2) the biological degradation of VOCs was found to produce 0.38 g of biomass per g of organics (expressed as chemical oxygen demand [COD]) degraded, which means that for a typical assembly plant, the estimated production of biomass would be about 0.5 to 1.5 times that of the paint sludge produced at the plant; 3) of the four paint VOCs fed to the reactor,practically all of the relatively hydrophilic VOCs (n-butanol,MEK, and BC) were captured from the air and degraded, with>75% of the relatively hydrophobic VOC, toluene, captured and degraded (without any biological activity in the scrubber water,99+% of the toluene fed to the reactor would have escaped the scrubber system via the gaseous effluent, due to the high volatility and low aqueous solubility of toluene, because of its high Henry’s law constant); 4) the addition of PAC to the biological reactor system improved the VOC removal, especially the hydrophobic solvent, toluene, by adsorption (PAC can be produced by pyrolyzing the sludge, a mixture of the excess biomass produced and the overspray paint polymers captured, which will be discussed later.); and 5) the comparison of the biological VOC removal process with the adsorption/thermal oxidation process for typical passenger-car and truck assembly plants, with annual production of 200,000 vehicles, showed a comparable VOC removal efficiency, an order of magnitude lower capital costs(about US $1.5 million for biological VOC removal versus US $14 to 23 million for adsorption/thermal oxidation) and more than a factor of two lower operating and maintenance costs for the biological process. The main reason for the difference in the capital costs between the two processes was that the water scrubber system, which can be used as a bioreactor, already exists in every assembly plant.
Biofiltration can also be easily used to treat exhaust spraybooth air containing paint solvents, as shown in many investigations[26-29], although the installation of a biofilter is expected to be costly to be effective because the flow rate of spraybooth air at an assembly plant is typically very high (>30,000 m3/min) [10], with a relatively low VOC inlet concentration (50 to 200 ppm) [10]. However, a biofilter may be an alternative to an RTO for destroying the VOCs in the desorbed air from a rotatingwheel adsorber after the volume of air to be treated has been reduced and the VOC concentration increased by the preceding adsorber.
A pilot biofiltration study was conducted by Boswell et al.[30] at a Toyota plant (Vancouver, BC, Canada) where aluminum wheels were painted. The VOCs in the spraybooth air were removed using a biological process, consisting of a biotrickling filter and separate biomatrix chamber (a biofilter). A removal efficiency of >85% was achieved on operating the process with an empty bed residence time between 15 to 35 seconds and under conditions of consistent temperature.
A factor to address when using any biological process for industrial applications is that of the organic loading, which tends to be intermittent and discontinuous due to the intermittent and discontinuous nature of manufacturing operations. Painting operations are no exception, are carried out intermittently,and stopped during weekends and for occasional shutdowns.A biological process (either a biofilter or a bioscrubber) can be operated with no loading for a relatively short period of time (e.g., up to a couple of weeks) [27], but will require supplementary food for a relatively long period of no loading. Dog food or organic waste solvents, such as spent purge solvents, could be used as the supplementary food.
Another factor to consider for biological treatment of scrubber water is the presence of nonbiodegradable dissolved organics in the water, which was found to be about 10 to 20% of the total COD at a Ford plant [31]. The nonbiodegradable organics did not contain paint solvents, which were all degraded, but the organics mostly consisted of nitrogen-containing organic polymers (more than 70%) and other organics. The nitrogencontaining organics were believed to have been derived mostly from paints and possibly from polymeric detackifiers. The other organics included silicon-containing compounds, which might have come from paint additives and maintenance chemicals used at the assembly plant from where the scrubber water was obtained. However, these organics did not affect the performance of the biological reactor when treating the scrubber water[6].
As with many industrial applications, nutritional requirements need to be carefully assessed for a biological treatment.The scrubber water used in painting operations was found to lack phosphorus [6] and; therefore, an appropriate amount of phosphorus needs to be added if the water is to be treated biologically. Some nitrogenous compounds were found in the water, possibly from the degradation of paint polymers, which could be available as a nutrient [6]. However, whether the nitrogen in the water is sufficient will need to be determined for a biological treatment.
Traditionally, pollution abatement in industrial sectors has been viewed as something that costs money, but has to be undertaken due to environmental regulations. However, this has changed as part of recent trends in “sustainable” and “green”manufacturing. In fact, pollution abatement can be achieved for not only reducing emissions, but also for saving money. There is an example relating to VOC-emissions control, where VOCs are captured and used for energy recovery. Recently, Ford developed a process for capturing VOCs from painting operations, with their conversion to electricity [15, 32]. This not only reduces VOC emissions, but also turns the gaseous pollutants into energy. In this process, the VOCs in the spraybooth air were concentrated using a fluidized-bed activated-carbon process, and reformed to produce hydrogen and carbon monoxide, with their conversion to electricity using a stationary fuel-cell system [15] or sterling engine [32].
The process was pilot-tested at a Ford assembly plant (Dearborn,MI, USA) [12, 15], which consisted of three processes: 1) a fluidized-bed activated-carbon adsorber; 2) a fuel reformer; and 3) a solid-oxide fuel cell. In the fluidized-bed adsorber, the VOCs in the exhaust air from painting operations were concentrated to increase the fuel value of the desorbed gas stream leaving the adsorber and entering the reformer. In the reformer, the VOCs were converted into a fuel mixture of mostly hydrogen and carbon monoxide, which was used to generate electricity via the fuel cell.
The fluidized-bed adsorber has been described previously in the VOC adsorption section, where the work by Anderson et al.[12] was introduced. The main reason for using the fluidized-bed busiprocess was to increase the VOC concentration factor from the 10-fold increase of a typical rotating-wheel adsorber to >1,000-fold via the fluidized-bed adsorber. Anderson et al. [12] observed a 3,000-fold increase in their pilot tests.
Reformation of the VOCs in the desorbed gas was carried out autothermally. Steam reforming was considered and tested, but not used due to the possibility of coking caused by the difficulty of maintaining a constant carbon-to-steam ratio and temperature at >800℃. The autothermally reformed fuel mixture was found to contain hydrogen (42%), carbon monoxide (8%), and inert gases (50%), which were mostly carbon dioxide and nitrogen,according to the test results.
Challenges were encountered during the pilot test: the variability in the VOC loading due to the intermittent nature of painting operations; large pressure differences between the three unit processes, the adsorber, the reformer, and the fuel cell; the variability in the VOC composition in the spraybooth air due to differences in the solvent make-up of the various paints used; and the high cost of fuel cells due to the emerging nature of this technology [15].
After the pilot-testing, Ford conducted a full-scale demonstration project at an assembly plant in Canada (Oakville, ON).For the generation of electricity (300 kW), a molten carbonate fuel-cell system was selected [33].
Traditionally, paint sludge has been collected and landfilled.However, attempts have been made to find a beneficial use for the sludge. Kim et al. [34] investigated the technical feasibility of converting paint sludge, a solid waste, into an activated-carbon like adsorbent, a useful product, by pyrolysis. They used paint sludge, dried black and white paints (as simulated paint sludge),a coal/paint mix, and a coal (as a reference base material) as source materials for pyrolysis. The pyrolysis was conducted at 600℃, using KOH as an activating agent, in a nitrogen atmosphere.The experimental results showed that: 1) the black?paint chars had substantially larger surface areas and adsorption capacities than the white?paint chars, probably due to the black pigment, carbon black, which is the basic ingredient of activated carbon, and the white pigment, titanium dioxide, which contributed to the large ash content of the white-paint chars;2) the paint?sludge char showed an adsorption capacity and ash content between those of the black?paint and white?paint chars, as expected due to the sludge having been formed from a variety of paints; 3) the paint-sludge char exhibited adsorption capacities between 5 and 20% that of a widely used commercial activated carbon; 4) the adsorption capacity of the paint-sludge char could be improved by the addition of supplementary carbonaceous material (e.g., coal); and 5) both the potential production and usage were estimated as being comparable, ranging from about 0.1 to 1 kg per vehicle. This could present attractive opportunities for recycling paint-sludge char, a waste product,for pollution abatement (e.g., the removal of VOCs from spraybooth air) and for vertically integrating vehicle products by recycling of the char into vehicle components (e.g., fuel-vapor carbon canisters and cabin-air filters).
The possibility of converting paint sludge into ceramic composites was investigated by pyrolyzing the sludge at either 600 or 1,000℃, under nitrogen and ammonia atmospheres [35, 36].These composites were found to contain crystalline CaTiO3, BaTiO3, TiO2, amorphous alumina and carbon. The composite,pyrolyzed under an ammonia atmosphere, was found to additionally contain a crystalline phase of titanium nitride. The possibility of using these composites as reinforcing materials was also demonstrated in the fabrication of metal-matric composites and reinforced plastic components.
Sanghvi and Massingill [37] developed a process for recovering automotive overspray paint materials, with their reconstitution into paint. The materials were recovered by removing the water and solvents via a vacuum treatment process, neutralizing the acid catalyst with a base, and removing contaminant particles through filtration. The recovered materials were then reformulated, with solvents and chemicals to replace those lost during the recovery operation, to have a solid content of 55%and VOC content of 0.36 kg/L. The reformulated paint was tested on metal panels, and showed reasonable properties for many industrial applications.
Paint sludge contains residual solvents and metals. When the sludge is not properly landfilled, some of these solvents and metals may leach and contaminate the water around the landfills.In an attempt to find a way of reducing the leaching potential of paint sludge, Arce et al. [38] investigated a solidification/stabilization process for the immobilization of the contaminants in an automotive, alkyd solvent-based paint waste prior to its disposal to landfill. The process involved the addition of lime,lime with coal fly ash, or lime with Portland cement to the paint waste, followed by exposing the lime-paint mixtures to carbon dioxide to produce calcium carbonate in the mixtures. Leaching tests on the stabilized mixtures showed good immobilization of the metal cations and partial immobilization of the Cl?, SO4 2?,and F? anions. However, only limited retention of dissolved organics was observed.
VOC emissions and their control from automotive painting were reviewed and have been discussed with respect to current and emerging painting operation practices. The topics included the nature of VOCs; the removal of VOCs by adsorption, absorption and biological processes; energy recovery from VOCs;and the beneficial use of paint sludge. The painting technologies currently used and being developed include electrolytic coating,water-based paints, solvent-based paints and powder paints.One of the main reasons for developing and improving these technologies has been to reduce VOC emissions. Of these technologies,the use of solvent-based paints results in the greatest VOC emissions, and they are expected to be widely used in the foreseeable future. Therefore, controlling VOC emissions from automotive painting is an important issue, which also needs to receive close attention. In this paper, the current practices and recent advances in VOC control at automotive assembly plants have been summarized.
There are nontechnical issues that many environmental engineers face when a new technology is developed and introduced as a replacement of an existing energy-intensive and costly process. The successful implementation of such new technologies often requires more than just their being technically and economically feasible and environmentally friendly.These issues that need to be resolved for the successful implementation include the inertia of past practices (attitude of “We have been doing it this way for many years. Why change?”), busiprocess Associaness interests with respect to old-technology suppliers, lack of system thinking (controlling individual waste streams without knowing their impact on the overall system), and moving targets(developing new technologies, while their targeted processes and products, as well as related environmental regulations keep changing).
![Solvent usage rates at typical Ford plants in North America [6]](http://oak.go.kr/repository/journal/10316/E1HGBK_2011_v16n1_1_t001.jpg)