Biodiesel derived from vegetable oils and animal fats has recently gained interest as a possible alternative to conventional fossil diesel due to the rapid decrease in fossil fuel reserves and the increase in crude oil prices. Biodiesel is a biodegradable, renewable and nontoxic fuel and its application as a diesel engine fuel improves exhaust emissions, with great potential to decrease environmental pollution [1, 2].
The vegetable oils used as a source for the production of biodiesel are mainly edible oils, such as soybean oil, rapeseed oil, palm oil, sunflower oil and cotton seed [1-3]. Because of the high prices of edible oils; however, non-edible oils, such as
The main methods for the production of biodiesel are direct use or blending of vegetable oil with solvents and physicochemical methods, such as micro-emulsification, pyrolysis and transesterification [1]. However, biodiesel is commonly formed from the triglycerides in vegetable oils via transesterification with the use of a monohydric alcohol for the conversion process in the presence of a basic or acidic catalyst. Glycerol is also generated as a major by-product from biodiesel production. The fuel-grade biodiesel produced from the transesterification process of vegetable oils has quite similar main characteristics to conventional fossil diesel [7].
The transesterification reaction is influenced by several experimental parameters, such as molar ratio of alcohol to oil, catalysts type and concentration, as well as the reaction conditions.In order to improve yields of biodiesel and the reaction rate, the following experimental parameters have to be considered; the molar ratio of alcohol to oil is one of the most important parameters capable of affecting the yield of biodiesel[8-10]. Excess alcohol is usually used to shift the equilibrium of the reaction in the direction of the product and to achieve a higher yield from the transesterification of vegetable oil [6]. If the molar ratio is constant, a similar yield of biodiesel will be obtained, regardless of vegetable oil used [11]. The molar ratio is correlated with the type and concentration of catalyst [12]. The transesterification with a basic catalyst is generally much faster than that with an acidic catalyst, but the reaction catalyzed by an acid catalyst results in a very high yield of biodiesel [12]. If the vegetable oil has a relatively high free fatty acid content and more water, the use of an acid catalyst in the transesterification is suitable [1,11]. The catalyst concentration is determined by the differences in the molar ratio, catalyst type and oil characteristics considered in the reaction process [12]. The reaction temperature is an important parameter affecting the reaction rate and yield in biodiesel production. Transesterification is usually carried out within the temperature range of 25 to 120℃, depending on the type of catalyst and oil used [1, 11-13].
However, the optimal conditions of various parameters in most experimental practices for the production of biodiesel have so far been individually determined by trial and error. Therefore, much times and efforts would be needed to investigate the effects of all combinations of parameters included in experimental research.
The objective of this study was to optimize the control parameters, such as catalyst type, catalyst concentration, molar ratio of alcohol to oil and reaction temperature, on the transesterification to produce rapeseed methyl ester. Using an experimental design technique by adopting the Taguchi approach (Taguchi method), with a set of orthogonal arrays, the optimal combination of experiment parameters was systematically estimated from the results of nine experimental runs, which had the equivalent effects of 81 experimental runs.
The rapeseed was provided by Daejeon, Korea, and dried in drying oven at 80℃ for 24 hours. The rapeseed oil was extracted from raw seeds, using a small screw press and filtered, before the transesterification without any treatment. All reagents for the transesterification reaction were purchased from Sigma (USA) and of analytical grade or higher. The standard for the fatty acid methyl esters (FAMEs), which was a Grain FAME mix (10 mg mL-1 of the FAME reference standard mix in methylene chloride), was purchased from Supelco (USA). Water, which was distilled and deionized using an ultra-pure system (resistivity = 18.3 MΩ cm), was used in all experiments.
2.2. Fatty Acid Composition of Rapeseed Oil and Analysis
In order to determine the fatty acid composition of the rapeseed oil, it was transesterified with 0.5 M sodium hydroxidemethanol and 14% boron trifluoride-methanol. After transesterification, the fatty acid composition of the rapeseed methyl ester was determined using gas chromatography (Fison 8000 series;Italy), equipped with a flame ionization detector and a HP-Innowax capillary column (30 m × 0.25 mm × 0.25 ㎛). The injector and detector temperatures were 240 and 280℃, respectively. The injector was used in the split mode, with a 50:1 split ratio. The oven temperature was programmed to ramp from 140 to 260℃ with 4℃ min-1, and then was held at 260℃ for 10 minutes. Nitrogen was used as the carrier gas, at a flow of 0.5 mL min-1.
For calculating the molecular weight of rapeseed oil, the average fatty acid molecular weight was calculated from its composition ratio and molecular weight. The rapeseed oil was assumed to be a triglyceride with three fatty acid chains of identical molecular weight. The molecular weight of the triglyceride was determined using the number of constituent atoms and its structure bonded to the oxygen atom of glycerol.
2.3. Production of Rapeseed Methyl Ester
The methyl ester was prepared by mixing methanol with rapeseed oil using several catalysts. The reactions were performed in a 500 mL three-necked batch reactor, equipped with a water-cooled reflux condenser, a mechanical mixer and a stopper to remove samples. This reactor was immersed in a constant-temperature water bath, which was capable of maintaining the reaction temperature to within ±1℃. The solid catalyst was dissolved in methanol, and the rapeseed oil added with stirring. A continuous mixer for converting input power into mechanical energy was used to mix the reactants. The impeller of mixer, which was based on a rotor-stator design, was kept at a constant 300 rpm. This was especially useful for mixing the catalyst-methanol solution and rapeseed oil. The reflux condenser was installed on the top of the reactor to capture and return any vaporized methanol.
After transesterification, the reaction mixture was transferred to a separating funnel equipped with a drainage valve at the bottom.The reaction mixture was allowed to settle for 24 hours to separate the methyl ester and glycerol. After settling, the glycerol layer was removed. The separated methyl ester was treated with a few drops of phosphoric acid (0.1 wt %) to deactivate the catalyst residues. Excess methanol was then distilled off under reduced pressure, with the methyl ester washed three times with warm distilled water (about 40℃) to remove the soap and residual methanol. Anhydrous magnesium sulfate was used to remove any residual water from the methyl ester, and subsequently removed by filtration.
2.4. Design of Experiment for the Optimization of Transesterification of Rapeseed Oil
The design of experiment used a statistical technique to investigate the effects of various parameters included in experimental study and to determine their optimal combination. The design of the experiment via the Taguchi method uses a set of orthogonal arrays for performing of the fewest experiments. That is, the Taguchi method involves the determination of a large number of experimental situations, described as orthogonal arrays, to reduce errors and enhance the efficiency and reproducibility of the experiments. Orthogonal arrays are a set of tables of numbers, which can be used to efficiently accomplish optimal experimental designs by considering a number of experimental situations [14].
An experimental design methodology adopting the Taguchi approach was employed in this study, with the orthogonal array
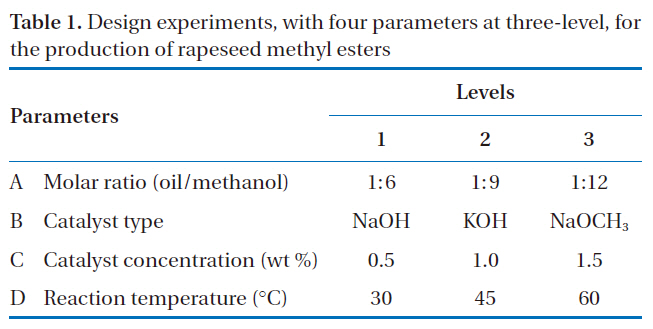
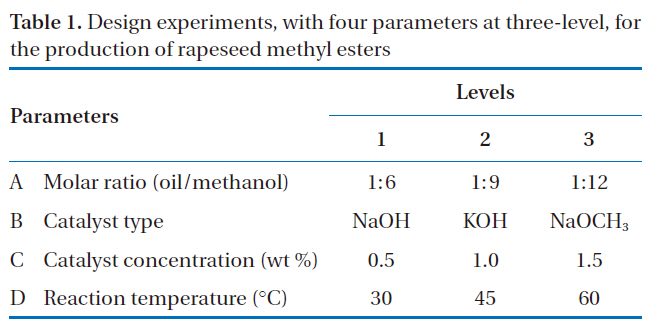
Design experiments with four parameters at three-level for the production of rapeseed methyl esters
design used to screen the effects of four parameters, including the molar ratio of alcohol to oil, catalyst type, catalyst concentration and reaction temperature, on the production of rapeseed methyl esters. The main operational parameters and levels were based on those from previously reported studies [11, 15-17]. The
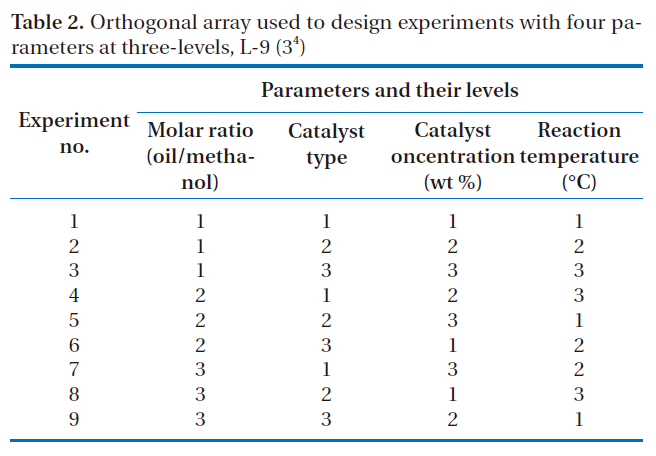
[Table 2.] Orthogonal array used to design experiments with four parameters at three-levels L-9 (34)
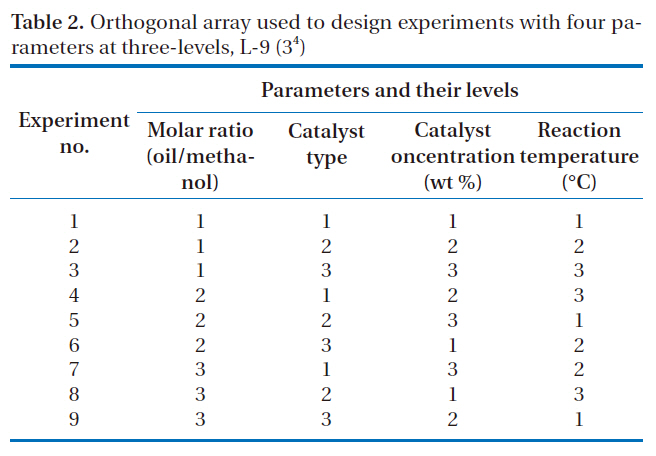
Orthogonal array used to design experiments with four parameters at three-levels L-9 (34)
four selected parameters, at three-levels, i.e. L-9 (34), experimentally studied are shown in Table 1. The diversity of factors was studied by crossing the orthogonal array of the control parameters,as shown in Table 2. L-9 refers to a Latin square and the experiment replication number. The numbers in Table 2 indicate the levels of the parameters.
In this study, QUALITEK-4, which is software for the Automatic Design and Analysis of Taguchi Experiments, was used to analyze the results and optimize the experiment conditions for setting the control variables.
3.1. Fatty Acid Composition of Rapeseed Oil
Vegetables oils, including rapeseed oil, are generally composed of several saturated and unsaturated fatty acids. The fatty
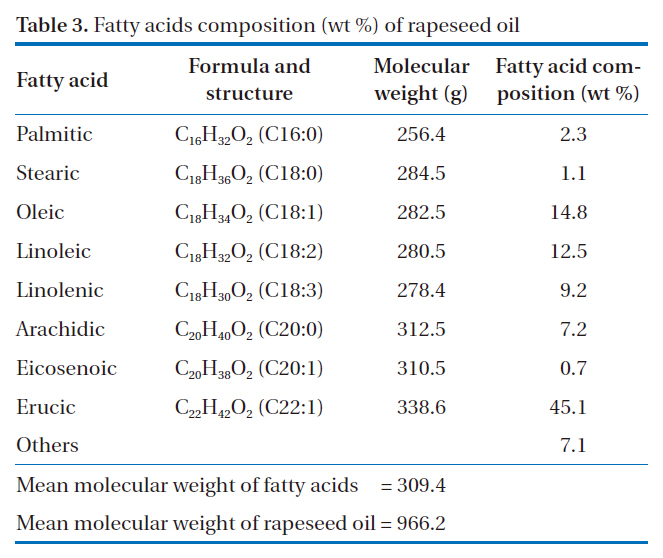
[Table 3.] Fatty acids composition (wt %) of rapeseed oil
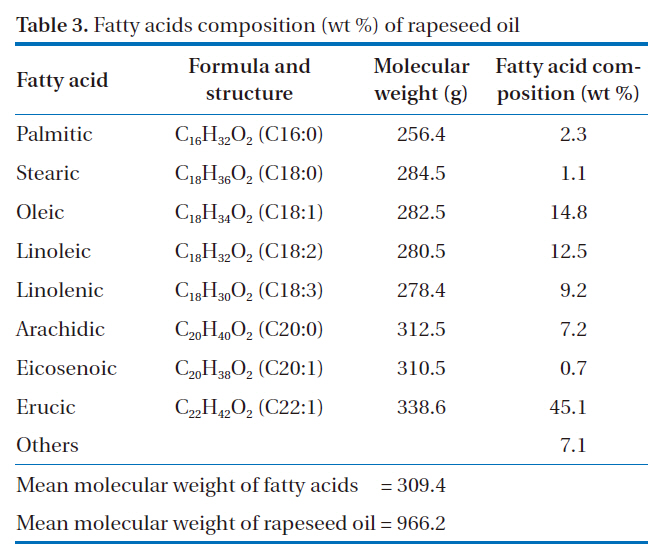
Fatty acids composition (wt %) of rapeseed oil
acids composition of rapeseed oil was analyzed according to previously described procedures, which are shown in Table 3. A total of eight different fatty acids were found in the rapeseed oil. The most dominant fatty acid was erucic acid (C22, one carbon bond), at 45.1 wt %, and also had major contents of unsaturated fatty acids, such as oleic acid (14.8 wt %) and linoleic acid (12.5 wt %) (C18, one and two double carbon bonds). Saturated fatty acids, such as palmitic (C16, saturated) and stearic (C18, saturated), were found in small amounts in this oil.
Rapeseed oil is made up of a mixture of triglycerides, which have chains of three fatty acids that contain about 16 to 22 carbon atoms, but with different degrees of saturation, as shown in Table 3. The molecular weight of a triglyceride would approximate to the value for rapeseed oil. The molecular weights of fatty acids were calculated by multiplying the numbers and molecular weights of carbon, hydrogen and oxygen atoms in the molecular.Multiplying the molecular weights of fatty acids by the ratio of the fatty acids composition to the total fatty acids, the value for rapeseed oil was obtained from the sum of the values of the individual fatty acids. The mean molecular weight of fatty acids was calculated to be 309.4 g, as a shown in Table 3. This was based on the assumption that the three fatty acids had the same molecular weights and a structure combined to the glycerol OH.Consequently, the calculated mean molecular weight (weights of one mole) of rapeseed oil (triglyceride) was 966.2, including the molecular weight of CH-C-CH (38 g). It seems likely that this value was higher than the values of other vegetable oils, due to the high composition ratio of erucic acid (45.1%), which has a higher molecular weight than that of other fatty acids.
3.2. Determination of Optimal Experimental Condition by the Design of Experiment
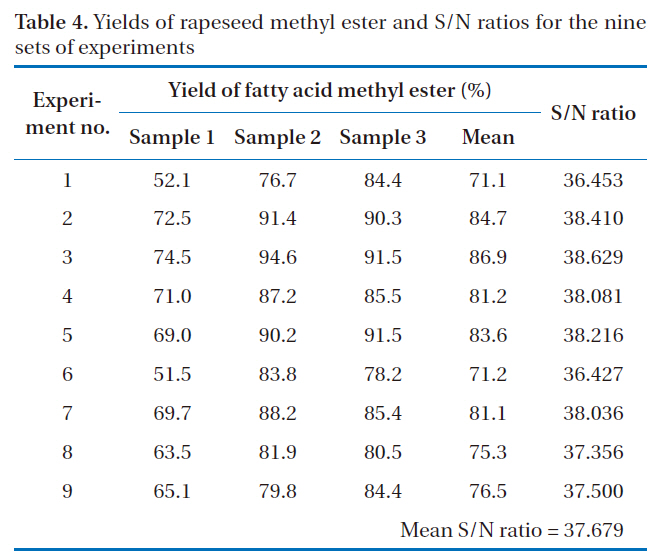
The yields of rapeseed methyl ester, as biodiesel, prepared under nine sets of experimental conditions are shown in Table 4. All experiments were performed with three repetitions, under the same experimental conditions (e.g., molar ratio of alcohol to oil, catalyst type, catalyst concentration and reaction temperature). From these results, experiment no. 3, which had a mean yield of rapeseed methyl ester of 86.9%, appeared to have the
[Table 4.] Yields of rapeseed methyl ester and S/N ratios for the nine sets of experiments
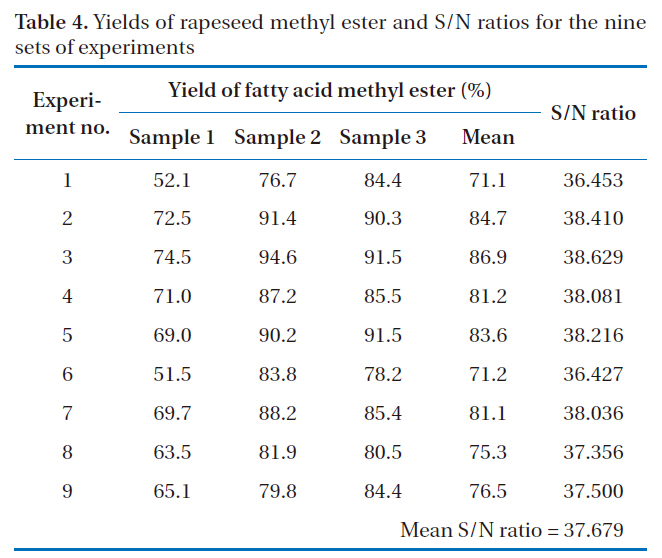
Yields of rapeseed methyl ester and S/N ratios for the nine sets of experiments
set experiment conditions with optimal parameters. Experiment no. 1 showed the lowest yield of rapeseed methyl ester, at 71.1%.However, it is likely that this would not be preferred way of selecting the optimal conditions using the Taguchi method for the design of an experiment.
In Taguchi method, the signal-to-noise (S/N) ratio is used to measure the quality characteristics deviating from the desired value. The S/N ratios are different in terms of their characteristics,of which there are generally three types, i.e. smaller-thebetter,larger-the-better and nominal-the-better [14].
According to the analysis for the case of ‘larger-the-better’, the mean squared deviations (MSD) of each experiment were evaluated using the following equation:
where
The S/N ratios for the nine sets of experiments are also shown in Table 4. The mean yield of rapeseed methyl ester and the S/N ratio were 79.1% and 37.679, respectively. Experiment no. 3 gave the highest mean yield of rapeseed methyl ester and had the largest S/N ratio. The relationship between the yield of rapeseed methyl ester and the S/N ratio was also similarly observed in other experiments.
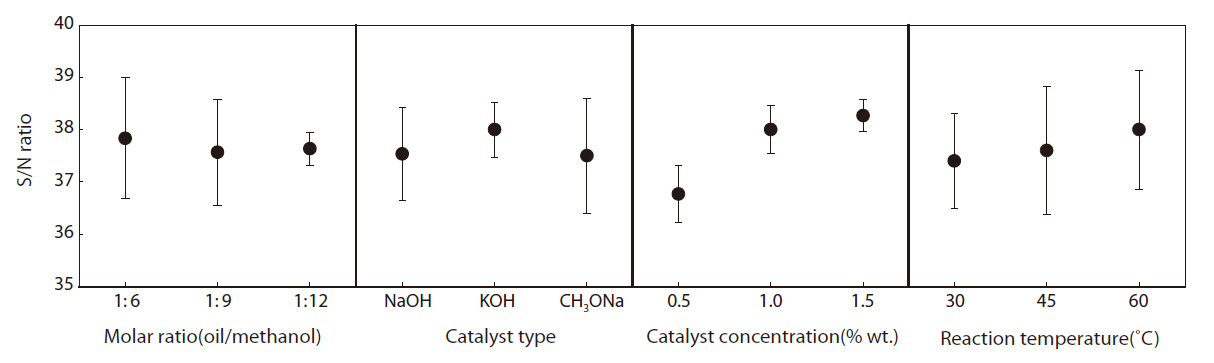
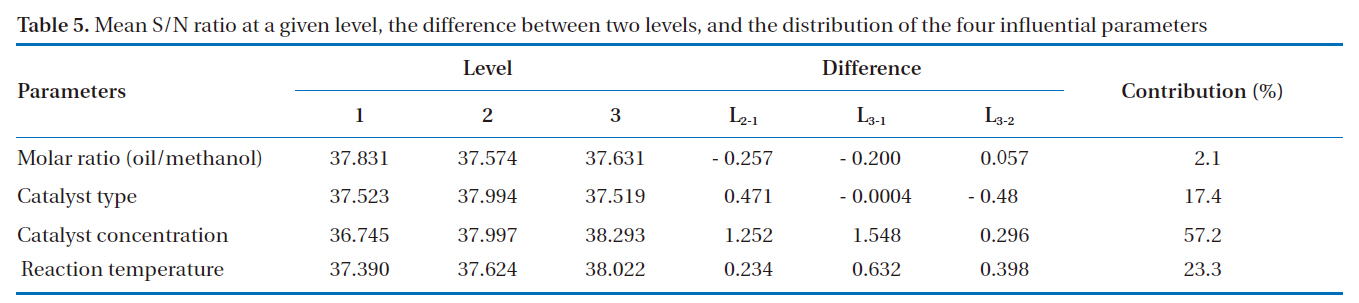
The mean S/N ratio, which was calculated from the effect of the parameters and the interactions at assigned levels, was the
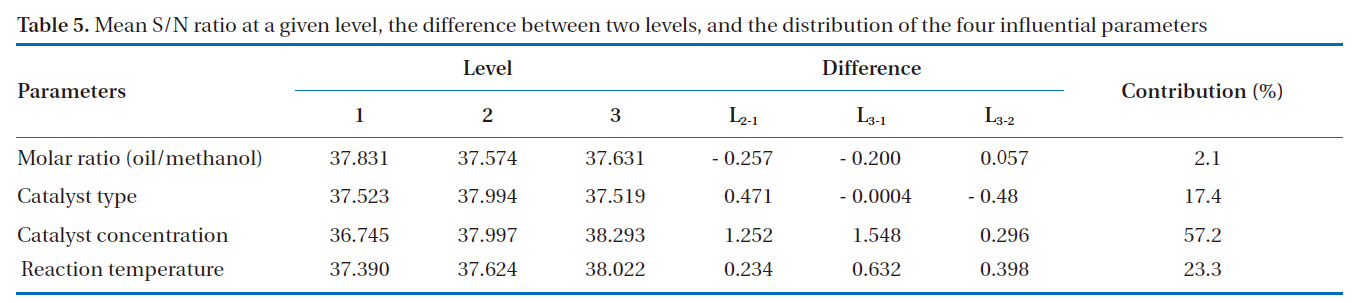
Mean S/N ratio at a given level the difference between two levels and the distribution of the four influential parameters
average of all the S/N ratios of a set of control parameters at a given level. For example, in the case of parameter A and level 1, the mean S/N ratio (37.831) was calculated using the values (36.453, 38.410 and 38.629) from experiment Nos. 1 to 3. In the case of parameter A and level 2, the mean S/N ratio (37.574) was calculated using the values (38.081, 38.216 and 36.427) from experiment nos. 4 to 6, and so on. The mean S/N ratio, the difference in two levels, and the distribution for the four influential parameters are summarized in Table 5. The contribution of an experimental parameter was calculated from the maximum difference in the values between the mean S/N ratios at each level. The order of influence of the parameters in terms of the yield of rapeseed methyl ester was: C (catalyst concentration) > D (reaction temperature) > B (catalyst type) > A (molar ratio).
The effects of individual parameters at different levels on the yield of rapeseed methyl ester are shown in Fig. 1. A larger mean S/N ratio indicates a greater effect of the control parameter at that level on the yield of rapeseed methyl ester; the catalyst concentration was the most influential parameter on the yield of rapeseed methyl ester. The greatest increase in the S/N ratio on the yield of rapeseed methyl ester was achieved from 0.5 to 1.0 wt % for the sample level; further increases were not significant above the 1.5 wt % sample level. Changing the oil/methanol molar ratio has a less relevant effect. The numerical value of the maximum point in each graph indicates the optimum range of the experimental conditions. Therefore, the optimum conditions for the greatest yield of rapeseed methyl ester were A1, B2, C3 and D3. In other words, based on the S/N ratio, the optimal parameters were A (oil/methanol molar ratio) at level 1 (1:6), B(catalyst type) at level 2 (NaOH), C (catalyst concentration) at level 3 (1.5 wt %) and D (reaction temperature) at level 3 (60℃).
In order to more systematically perform an analysis of the relative importance of each parameter, an analysis of variance (ANOVA) was used to optimize the results obtained using Taguchi method. This provided information on the relative influence of parameters and their interactions with respect to the various results. According to the ANOVA results, the most influential parameter on the production of rapeseed methyl ester was the catalyst concentration. The percentage contribution of each individual parameter was determined by the ratio of the pure sum to the total sum of the squares. The catalyst concentration had a percentage contribution of 77.582, which was larger than any other parameter, which was in agreement with the largest slope for the catalyst concentration in the graph of the S/N ratio (Fig.1).
3.3. Result of the Proposed Optimized Experimental Condition and Validation Studies
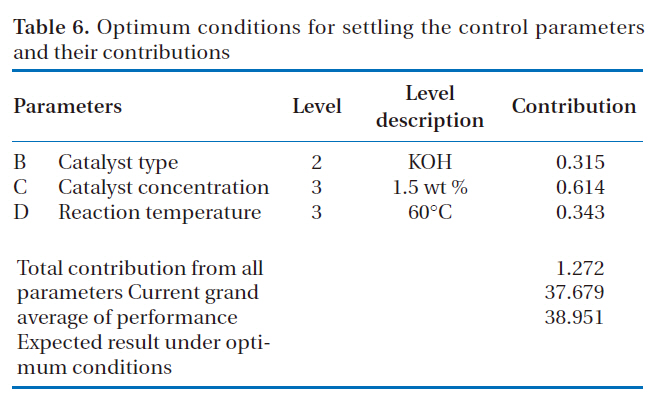
The optimum conditions to achieve effective performance for the production of rapeseed methyl ester and the contributions of each parameter to the S/N ratio under the optimal conditions are shown in Table 6. The catalyst concentration was
[Table 6.] Optimum conditions for settling the control parameters and their contributions
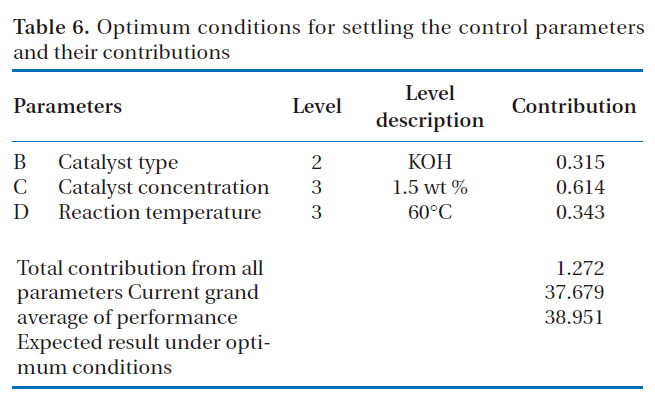
Optimum conditions for settling the control parameters and their contributions
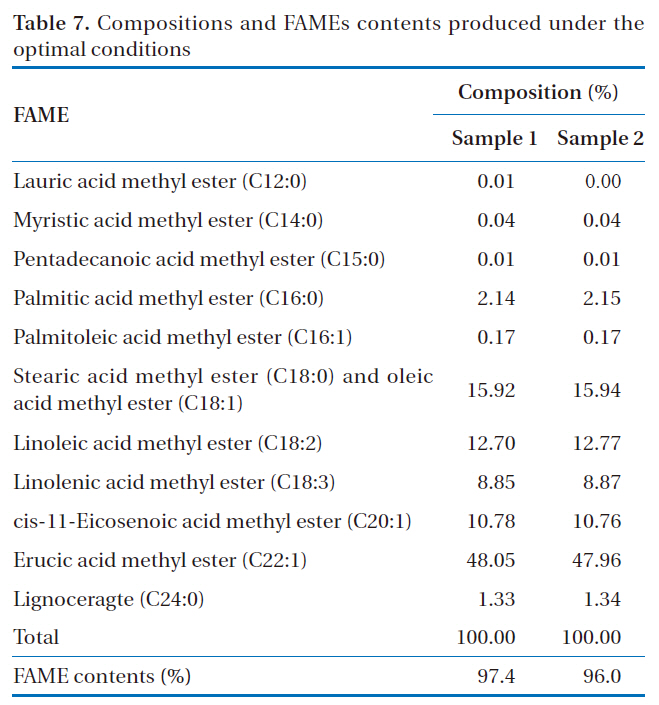
[Table 7.] Compositions and FAMEs contents produced under the optimal conditions
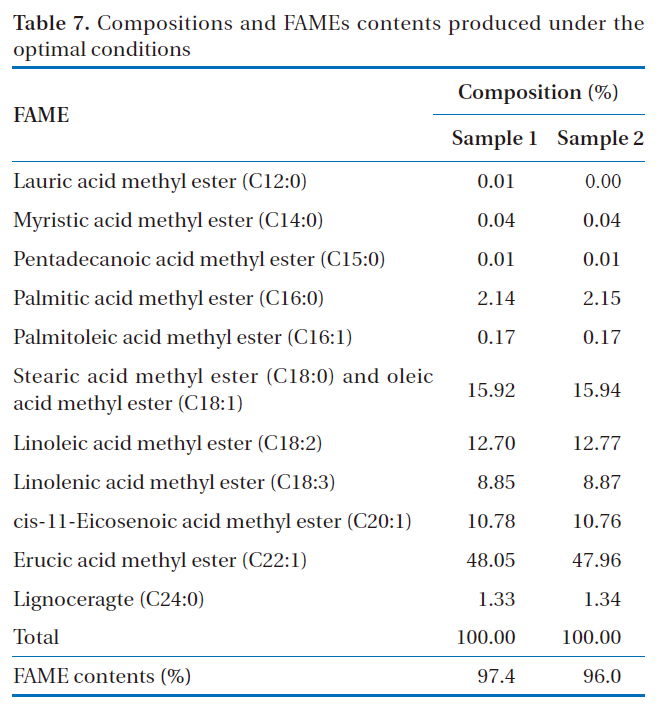
Compositions and FAMEs contents produced under the optimal conditions
found to be the most important parameter in influencing the production of rapeseed methyl ester and the parameter contribution under the optimized conditions was found to be 0.614. The catalyst type had the least impact of all the parameters studied, with a parameter contribution of 0.315.
The parameter contribution is defined as the degree of modification obtainable by setting the parameter to the desired level, which is determined by the difference between the S/N ratio of the optimal level for each parameter, shown in Table 5, and the mean S/N ratio, shown in Table 4. In the case of parameter B(catalyst type) at level 2 (potassium hydroxide) in Table 6, for example, the parameter contribution was determined by subtracting the grand average S/N ratio (37.679) from the average S/N ratio of parameter B at level 2 (37.994), which gave 0.315.
The maximum performance expected for the production of rapeseed methyl ester under optimum conditions was evaluated from the result expected for the S/N ratio under the optimum conditions. The expected theoretical yield of rapeseed methyl ester can be calculated using Eqs. (1) and (3).
The MSD calculated from the expected S/N ratio under the optimum conditions (38.951, as shown in Table 6) was 0.000127, and the expected yield of rapeseed methyl ester (
In order to confirm the optimal conditions derived from this study, the production of rapeseed methyl ester was duplicated under the same conditions. From the results shown in Table 7,the average yield of rapeseed methyl ester was 96.7%. This result was found to be high compared to the result obtained from experiment no. 3 (86.9%), which gave the highest yield of rapeseed methyl ester, and to the theoretically expected value (88.6%) mentioned above. This result showed significant enhancement of the process performance with the optimized parameters for the production of rapeseed methyl ester.
The Taguchi method, which uses a set of orthogonal arrays for performing the fewest experiments, was employed to design experimental trials, with an ANOVA performed to more systematically analyze the relative importance of each experimental parameter on the production of rapeseed methyl ester.
The catalyst concentration, catalyst type and reaction temperature were found to be significant parameters affecting the production of rapeseed methyl ester. The contribution of the catalyst concentration on the production process was larger than that of any other parameter. The yield of rapeseed methyl ester obtained with the optimal experimental parameters was greater than that obtained from experiment no. 3, which gave the highest yield from the experimental trials, and the theoretically expected value.
The experiments conducted under the optimized conditions showed a meaningful enhanced process performance. The Taguchi method provided a systematic and efficient mathematical approach to evaluate and optimize the process for the production of rapeseed methyl ester, using only a few well-defined experimental sets for the optimization of the design parameters.