Recently, many researchers have attempted to find or develop suitable materials and technology for the remediation of metal-contaminated soils. As a consequence, a number of plants with the ability to accumulate specific heavy metals have been identified, and the biochemical mechanisms relevant to accumulation and defense against heavy metal toxicity have been thoroughly assessed (Tong et al. 2004, Xu et al. 2009). The potential use of trees as a suitable vegetation cover for heavy metal-contaminated land has received an increasing amount of attention over the past 10 years. Trees have been identified as a low-cost, sustainable, and ecologically sound method for the remediation of heavy metal-contaminated land (Dickinson 2000). The use of native plants for phytoremediation is important because these plants are frequently better in terms of survival, growth, and reproduction under environmental stress conditions than plants introduced from other environments. nated, sediment-derived soils, with foliar samples being employed to evaluate metal bioavailability, as previously described by Vandecasteele et al. (2002, 2005). However, metal concentrations in willows depend heavily on species (Lunackova et al. 2003, Vandecasteele et al. 2004, 2005, Han et al. 2007), clone (Landberg and Greger 2002, Vandecasteele et al. 2005), growth performance (Klang-Westin and Perttu 2002), root density and distribution within the soil profile (Keller et al. 2003), and sampling period (Vandecasteele et al. 2004, 2005). Additionally, as willows are relatively easy to grow from cuttings and evidence high growth rates, they are rather well suited both to the testing of metal bioavailability and accumulation, and also to phytoremediation, which can be combined with energy production from biomass (Dimitriou et al. 2006, Lewandowski et al. 2006, Vervaeke et al. 2006, Meers et al. 2007).
This is an Open Access article distributed under the terms of theCreative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestrictednon-commercial use, distribution, and reproduction in any medium,provided the original work is properly cited.
In Korea, there are hundreds of closed and abandoned zinc mines, many of which have been contaminated by a variety of toxic metals, such as Cd, Zn, Pb, Ni, As, etc. In particular, Cd and Zn concentrations in mining soil have been measured at more than 30 and 2,600 mg/kg, respectively (Ministry of Environment 2009).
Cadmium, which is not essential to plants, and Zn, which is essential to plants, is elements with otherwise similar geochemical and environmental properties (Siedlecka 1995). The association of Cd and Zn in the environment and their relative chemical similarity result in interactions between Cd and Zn during plant uptake, as well as transport from roots to aboveground parts (Das et al. 1997). Studies undertaken to gain insight into the interaction between Zn and Cd have generated inconsistent results. White and Chaney (1980) previously reported that Cd uptake was reduced in both soybean roots and shoots via the application of Zn. Wu and Zhang (2002) previously noted that the physiological damage induced by Cd toxicity might be alleviated by the application of Zn. Eriksson (1990) determined that the Zn-Cd interaction varied with different crop species. Few studies, however, have been conducted thus far regarding the interaction of Cd and Zn in tree species grown in high metal-contaminated soil.
The overall objectives of this research were to gain insight into the Cd-Zn interaction in the context of Cd-Zn bioaccumulation of
>
Plant materials and treatments
Seven
After 120d of growth, the leaves, stems, and roots were carefully removed, then rinsed thoroughly twice with distilled water. After oven drying of the tissues at 70℃ to constant weight, the dry weights of the leaves, stems, and roots were recorded.
The dried leaves, stems, and roots (0.5 g each) were ground (< 1 mm) and used to determine the concentrations of Cd and Zn. Nitric acid (70%, 15 mL) and hydrogen peroxide (30%, 5 mL) were added to 0.5 g of dried, ground plant samples in a digestion vessel. The samples were digested using the microwave digestion system, cooled after the addition of distilled water, and filtered prior to analysis. Cadmium and Zn concentration in the digested tissues were measured using an atomic absorption spectrophotometer (AA-6701F; Shimadzu, Tokyo, Japan). In order to verify the accuracy of our determinations of Cd and Zn, standard reference materials of pepperbush (CRM No.1) and sargasso (CRM No.9) provided by the National Institute for Environmental Studies, Tsukuba, Japan were analyzed. The recovery rates of Cd and Zn with respect to their certified values were between 90% and 95%.
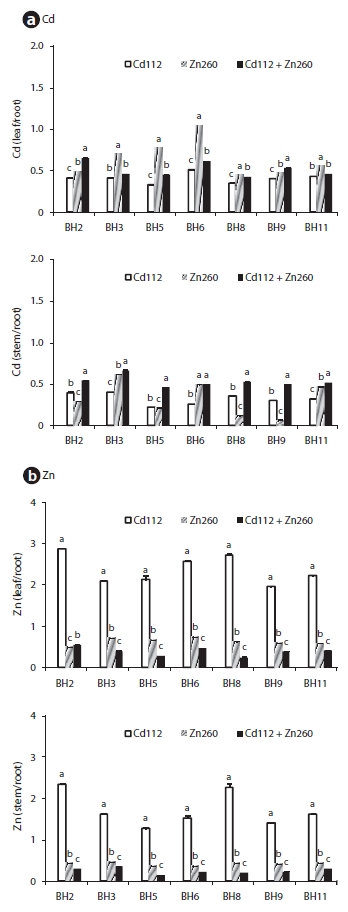
Leaf and stem translocation factor (TF), which is defined as the ratio of the concentration of a metal in leaf and stem to its concentration in the root, was used to estimate the plant's potential for use in phytoremediation purposes. The phytoremediation ability of the seven
The data were statistically analyzed using the SAS ver. 8.01 (SAS Inc., Chicago, IL, USA). Mean values per treatment were compared via GLM. When significant differences (
>
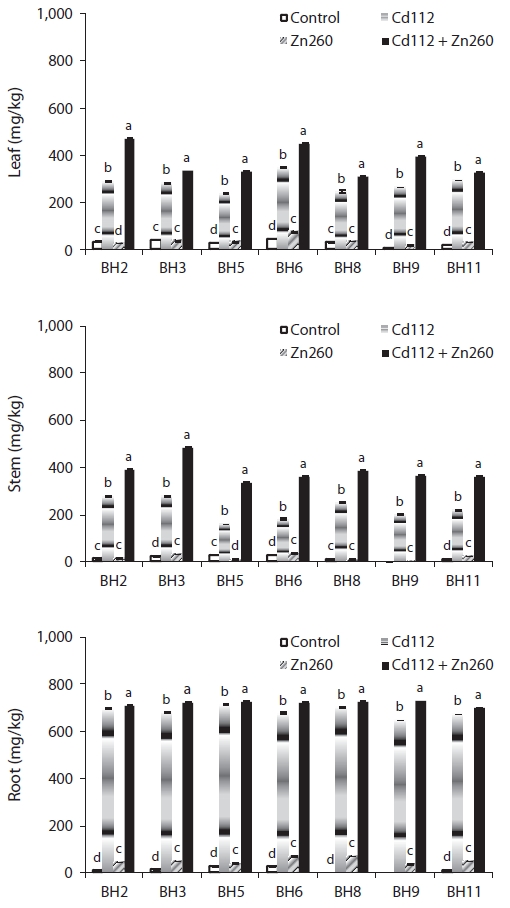
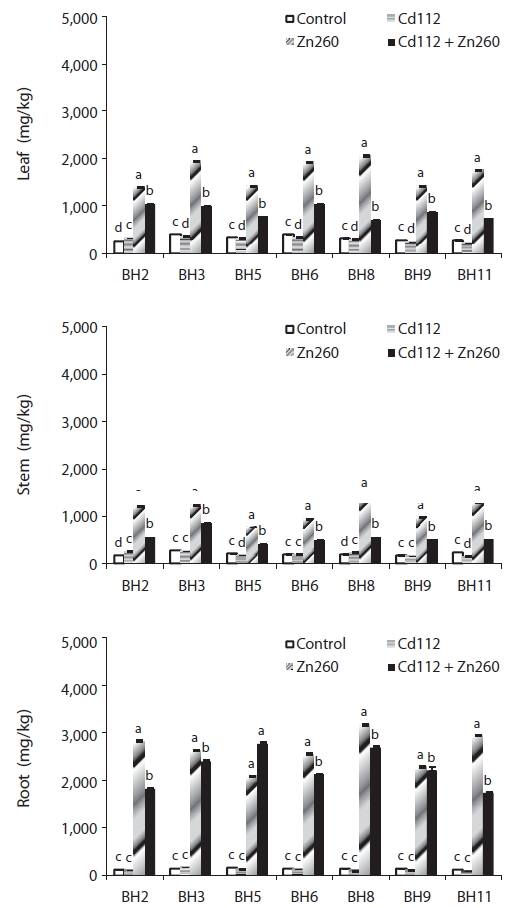
Cadmium and zinc concentration
In our study, we detected significant effects of Cd and Zn treatment, clones, and their interactions with Cd and Zn concentrations and TF (
The low mobility of Zn, unlike that of Cd, was not improved in the combined Cd and Zn treatment group. Leaf and stem Zn concentrations of clone BH8 in the combined Cd and Zn treatment group was reduced by 66% and 61%, respectively, relative to those in the Zn alone treatment group. Root Zn concentration of all clones in the combined Cd and Zn treatment were also reduced from 3% to 41% relative to the Zn alone treatment group (Fig. 2).
Metal concentrations in plants vary with the plant species (Alloway et al. 1990), and the uptake of heavy metals by plants from soil occurs either passively with the mass flow of water into the roots, or via active transport across the plasma membranes of root epidermal cells. Generally, heavy metal uptake by roots depends on both soil and plant factors (e.g. source and chemical form of elements in soil, pH, CEC, organic material, plant species and tissues, plant age, etc.).
The interaction between Cd and Zn in plant tissues is known to be remarkably variable, involving species-, population-, and exposure-dependent interactions (Eriksson 1990). For instance, some studies have demonstrated that Zn (or Cd) suppresses the bioaccumulation of Cd (or Zn) by reducing uptake (i.e., antagonistic interactions) (White and Chaney 1980, Hart et al. 2002), whereas other studies have demonstrated that Zn (or Cd) enhances the bioaccumulation of Cd (or Zn) by increasing uptake (i.e., synergistic interaction) (Dudka et al. 1994, Xu et al. 2009).
Generally, however, most of our findings may be summed up as follows: Zn reduces the uptake of Cd by both the root and foliar systems (Adriano 1986). White and Chaney (1980) previously noted that Cd uptake was reduced in both soybean roots and shoots via the application of Zn. In support of these results, Gomes et al. (2002) demonstrated that Cd uptake is mediated via the movement of a Zn-transporter protein across the plasma membrane in yeast cells. Zinc was also shown to interfere with phloem-mediated Cd transport in durum wheat, possibly by competition with Cd for the binding sites of a common transporter protein on the plasma membranes of sieve tube cells (Cakmak et al. 2000).
Unlike the results of a previous study (White and Chaney 1980, Adriano 1986, Nan et al. 2002), the interaction effects of Cd and Zn in the present work were found to be synergistic with Cd, in the presence of Zn strongly elevating leaf and stem Cd concentrations, but antagonistic to Zn, in the presence of Cd, which elevates leaf and stem Zn concentrations when exposed to high concentrations of Cd and Zn. Xu et al. (2009) reported that shoot and root Cd concentrations in the combined Cd and Zn treatment group with the addition of EDTA were increased by 18.6% and 391.9%, respectively, and shoot and root Cd concentrations were in the following order: Cd + Zn > Cd > Zn, in the presence of EDTA. Huebert and Shay (1992) suggested that the increases in shoot and root Cd concentrations in the combined Cd and Zn treatment group in the presence of EDTA may have been attributable to the application of synthetic chelators to contaminated soils, which can enhance the uptake and accumulation of heavy metals in plant shoots, and the interaction of Cd and Zn on the uptake of Cd and Zn by plants is affected by the presence of chelators such as EDTA.
In contrast to the observed increase in Cd concentration, the results of our present study demonstrated that leaf, stem, and root Zn concentrations were reduced in the combined Cd and Zn treatment group. Xu et al. (2009) noted that, after the addition of EDTA, shoot and root Zn concentrations were reduced in the combined Zn and Cd treatment group, and Zn concentrations were in the order of Zn > Cd + Zn > Cd in both the absence and presence of EDTA. Based on these results, Xu et al. (2009) proposed that the presence of Cd in soil inhibited the uptake of Zn by plants.
The patterns of cadmium and Zn of the seven clones are provided in Fig. 3. In the Cd alone treatment group, the leaf Cd TF ranged from 0.33 (clone BH5) to 0.51 (clone BH6) and the stem Cd TF ranged from 0.22 (clone BH5) to 0.40 (clone BH3) (Fig. 3). In the Zn alone treatment group, leaf Zn TF ranged between 0.48 (clone BH2) and 0.74 (clone BH6), and the stem Zn TF ranged between 0.36 (clone BH5) and 0.46 (clone BH3). Although the TF of Cd and Zn from root to leaves was higher than from the roots to the stems, the value was still less than 1. This implies that the accumulation of Cd and Zn in the roots was more profound than in the leaf and stem. In a manner similar to that of the Cd concentration, the combination Cd and Zn treatment stimulated the translocation of Cd from the roots to the leaves and from the roots to the stem, whereas it suppressed the translocation of Zn from the root to the leaf and stem. Leaf Cd TF in clone BH2 and stem Cd TF in clone BH5 were increased by 59.4% and 106.7%, respectively, relative to the Cd alone treatment group. However, the leaf Zn TF in clone BH9 and the stem Zn TF in clone BH5 were reduced by 59.7% and 58.7%, respectively, relative to the Zn alone treatment group. Interestingly, however, the leaf Zn TF in clone BH2 was increased in the combined Cd and Zn treatment group. The observed increase in the leaf Zn TF in clone BH2 indicated that the reduction in the leaf Zn concentration (25%) was lower than that of the root Zn concentration (35%) in the combined Cd and Zn treatment group.
The reason for these differences between the previous results (White and Chaney 1980, Adriano 1986, Nan et al. 2002) and those of the present work may be the result of different Cd and Zn concentrations and their combinations in soils, as well as the different characteristics of the soils and the different plant species and tissues utilized in these studies (Huebert and Shay 1992, Xu et al. 2006).
Therefore, the results of this study indicate that in soils contaminated at high levels with Cd and Zn, soil Zn should not be expected to reduce Cd significantly in plant parts, although there a strong antagonistic Zn effect on Cd accumulation has been noted in plant tissues. Moreover, our findings are generally consistent with those reported by Smilde et al. (1992), Moraghan (1993) and Zhou et al. (1994). Smilde et al. (1992) concluded that Cd and Zn were synergistic to some degree, with plant Zn uptake increasing with the application of Cd on the basis of interaction experiments conducted in pots filled with loam soil. Moraghan (1993) reported that the Cd-Zn effects were synergistic in the presence of added Cd, and that the accumulation of Cd in flax seed was reduced by added Zn in the absence of added Cd. Zhou et al. (1994) concluded from their experiment that the interaction between Cd and Zn resulted in an increase in Cd accumulation and a reduction in Zn uptake in the rice plant.
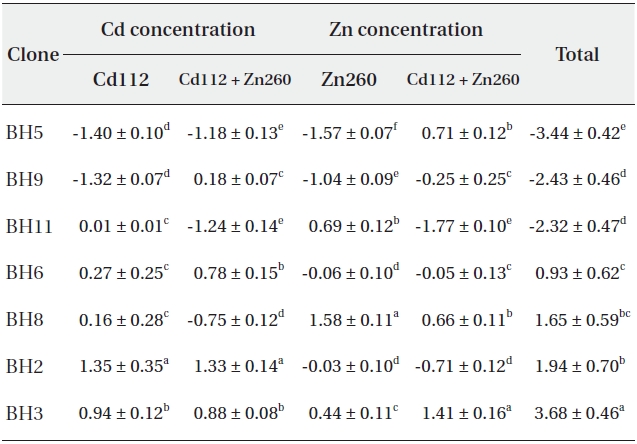
Based on our results, it would be difficult to select the optimal material for phytoremediation, owing to the complex interaction between Cd and Zn. Thus, in an effort to select the optimal clone for Cd and Zn phytoremediation, the phytoremediation potential of the seven clones studied herein was ranked on the basis of the total sum of standard indices calculated from the Cd and Zn concentrations in Cd and Zn alone and in the combined Cd and Zn treatment group (Table 1). As a consequence, clone BH3 evidenced the most profound (SI = 3.68) phytoremediation potential, and clone BH5 evidenced the lowest (SI = -3.44).
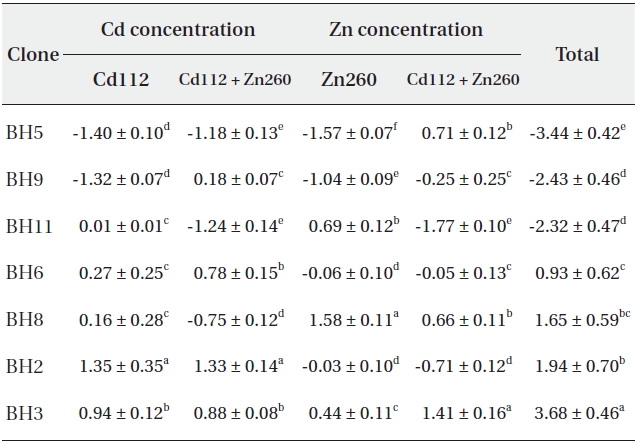
Standard indices on Cd and Zn concentration of seven Salix caprea clones treated with Cd or Zn alone or the combined Cd and Zn
In summary,