This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons. org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
can survive under the extremely harsh conditions pertaining in arid zones, from temperatures of -25 to 50°C. H. aphyllum regenerates by seeding. The seeds begin to mature from late October until the first days of January. H. aphyllum seeds begin to germinate in these deserts in late February, and its leaves appear in May. In arid environments, the seedling is the most vulnerable stage in the plant life cycle, and germination determines when seedling growth begins (Gutterman 1993). Seed germination performs an important function in the regeneration of plant species, particularly under arid and unpredictable environmental conditions, most notably those of Mediterranean ecosystems (Gimmenez-Benavides et al. 2005). The germination responses of species to environmental parameters determine their distribution in arid environments. Several environmental factors function as determining factors in germination (Bewley and Black 1994); seed germination is affected by light, temperature, and a host of other environmental factors (Bewley and Black 1994, Baskin and Baskin 1998). Among these factors, temperature is a crucial factor governing the maximum germination percentage (Heydecker 1977) and germination rate (Flores and Briones 2001, Phartyal et al. 2003). It has been recognized since as early as 1860 that three cardinal temperatures (minimum, optimum, and maximum) described the temperature range over which the seeds of a particular species can germinate (Bewley and Black 1994). Seeds of each species possess the capacity to germinate over a defined temperature range, which is referred to as the cardinal temperature (Alvarado and Bradford 2002). Finally, the cardinal temperature (minimum or basic temperature, optimum, and maximum) is the temperature range within which the seeds of a particular species can germinate. The minimum cardinal temperature is the lowest temperature at which crop growth can occur; this temperature is referred to as the base temperature (Tb), and no growth occurs below that temperature. The optimum cardinal temperature (To) is the temperature at which crop growth and performance are at their maximum. Finally, the maximum cardinal temperature (Tm) is the highest temperature at which plant growth can occur (Alvarado and Bradford 2002). Finally, growth and development processes are optimal when the temperature is between the minimum and maximum bounds of the range, and close to the optimum cardinal temperature. There are clear minimum and maximum temperatures for germination, and within the broad range between them all seeds can germinate. Seed germination begins at the minimum temperature and the germination rate increases with increasing temperature to the optimum, and then decreases with further rises in temperature to the maximum (Bewley and Black 1994). Some methods have been developed previously to describe species growth data (e.g. germination percentage, germination rate) in response to temperature, but among those methods the germination rate method is the most specifically salient to temperature (Covell et al. 1986), and a great deal of previous research has been done in efforts to understand these relations; regression analysis is generally recognized as the best statistical tool for the investigation of relationships among these variables. The data collected at sub-optimal and supra-optimal temperatures were used to construct two linear regressions to describe the increases and decreases in the germination rate at sub-optimal and supra-optimal temperatures, respectively. The optimum temperature is the temperature at which these two lines intersect (Covell et al. 1986).
Roberts (1988) previously described a model elucidating the relationship between germination rate and temperature. The influence of temperature on germination rate and thermal time in plant species has been previously evaluated by a variety of researchers, including the common crupina (
Mature seed lots of
After eliminating humidity, the seeds were stored in bags in a refrigerator (5℃) until the beginning of each experiment. This study was conducted at the Department of Desert Region Management, College of Agriculture, University of Shiraz, Iran. The seeds were treated with 0.2% Benomil fungicide prior to the germination test. In all experiments, four replications of 50 seeds of two provenances were sown at 5, 10, 15, 20, 25, and 30 on Whatman No. 1 filter paper, in Petri dishes (50-mm diameter) under light conditions (Ghaedi et al. 2009). The filter paper was moistened with approximately 5 mL of demonized water, allowing about half of the seeds to be immersed in the solution. During this experiment, lost water was replaced when necessary. The seeds were considered to have germinated when the emerging radical was over 5-mm long (Young et al. 1981). The numbers of germinated seeds were recorded on a daily basis. After nine days, the final number of germinated seeds was calculated, as well as the percentage of germination. The mean time to full germination was calculated in accordance with the equation developed by Ellis and Roberts (1981). The germination rate was calculated by the inverse of mean time to full germination (Tobe et al. 2000, Flores and Briones 2001).
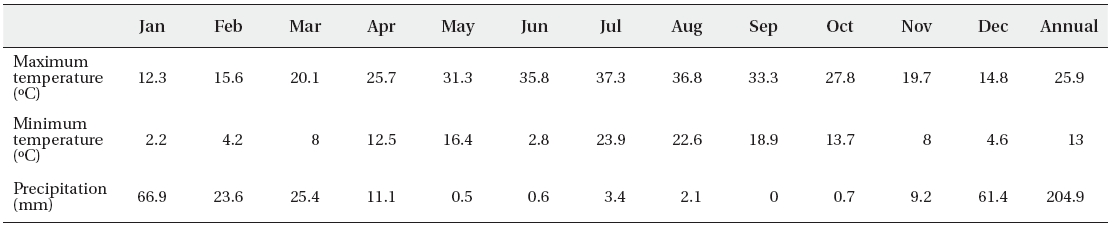
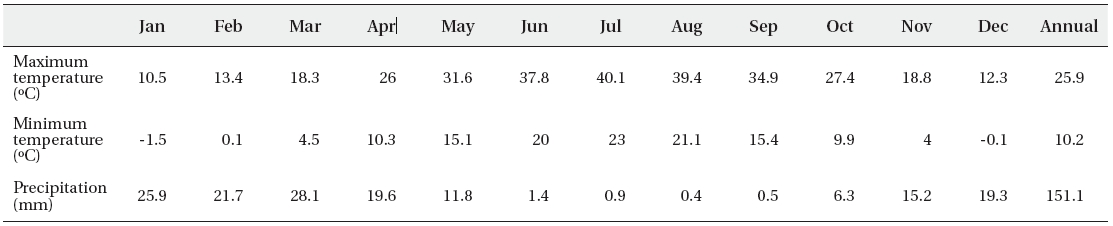
Average of maximum, minimum of temperature and monthly average of total of precipitation of the Fars dune desert
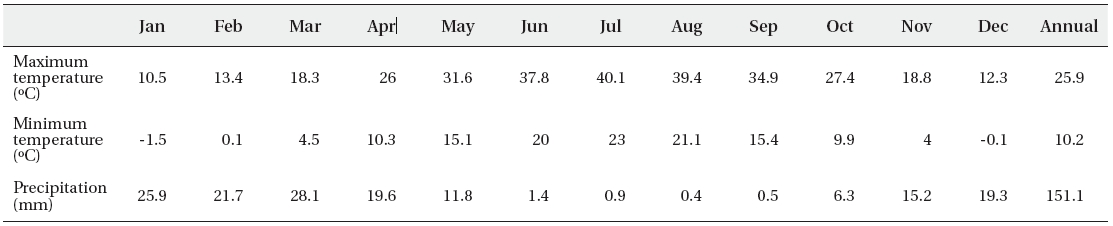
Average of maximum, minimum of temperature and monthly average of total of precipitation of the Qom dune desert
GR = 1/MTG
GR: germination rate
MTG = ∑ (ni.ti)/∑ n
MTG: mean time to full germination
n: number of seeds newly germinating at time t
t: number of day from sowing
Cardinal temperature was calculated on the basis of the responses of germination rate to temperature (Villalobos et al. 2002). We employed an intersecting-line model (Hadley et al. 1983, Kocabas et al. 1999, Phartyal et al. 2003, Al-Ahmadi and Kafi 2007). The T base and T maximum were derived from the intersection of each regression line with the abscissa, and the T optimum was calculated from the intersection of two linear regression lines of the germination rate at sub-optimal and supra-optimal temperatures (Covell et al. 1986).
Data were checked for normality and then analyzed using MSTATC statistical software (MStatC Inc., East Lansing, MI, USA). Treatment means were separated by Duncan's test in cases in which the F value of the treatments was significant at probability levels of 0.05 or 0.01.
The germination rate differed significantly among temperature treatments (
[Table 3.] Equation calculated cardinal temperature for Haloxylon aphyllum L. seeds

Equation calculated cardinal temperature for Haloxylon aphyllum L. seeds
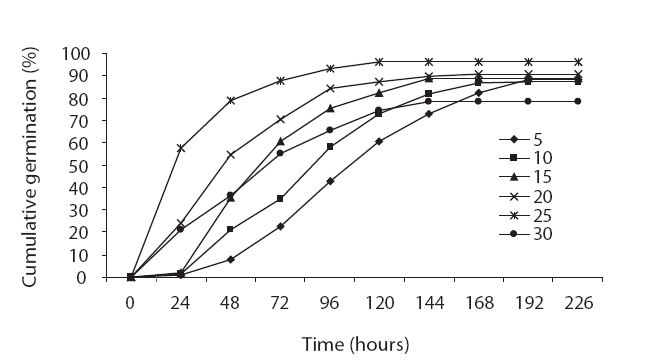
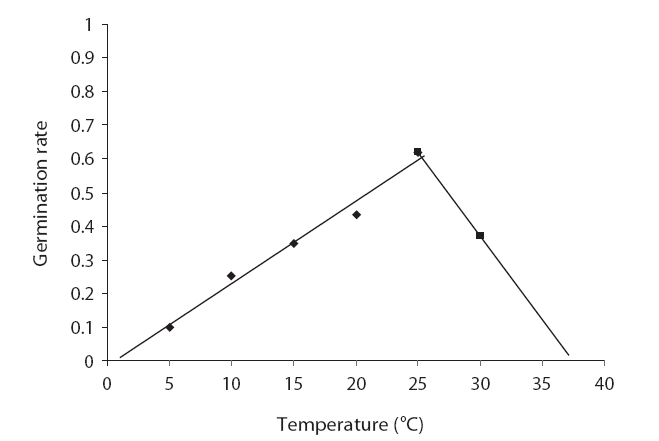
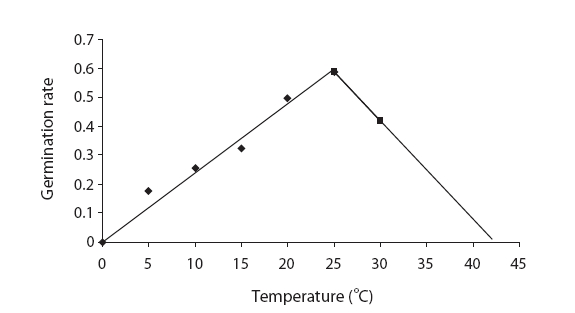
The germination rate evidenced an increasing trend from 5℃ to 25℃ and the germination rate decreased abruptly with increases in temperature. The highest and lowest germination rates were noted at 25℃ and 30℃, respectively (Fig. 2). The germination rate was 0.1 at 5℃ and reached 0.62 at 25℃, and then decreased to 0.37 at 30℃ (Fig. 2). The drawn-to-fit linear scale to cumulative normal distribution evidenced a positive slope from 5℃ to 25℃ and a negative slope from 25℃ to 30℃. The intersection of fit linear regressions between 5℃ to 25℃ and between 25℃ to 30℃ with the abscissa showed a T base of 0.62 and a T maximum of 37.9, respectively (Table 3). The intersection of two linear regression lines of the rate of germination at sub-optimal and supra-optimal temperatures revealed the optimal temperature (25.69) (Table 3).
>
Fars dune desert seed source
The germination rate was shown to be affected significantly by the temperature treatments (
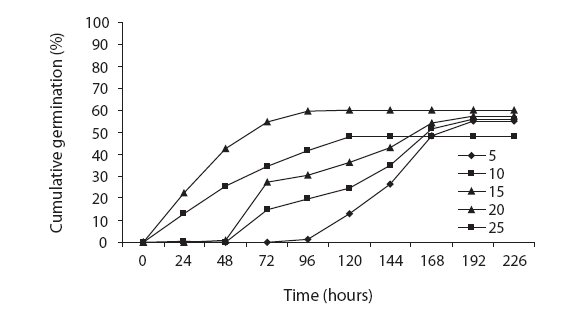
The germination rate increased with increasing temperature. The highest seed germination rate (0.5) was obtained at 25℃. The germination rates were 0.1 and 0.5 at 5℃ and 20℃, respectively, and decreased to 0.3 at 25℃ (Fig. 4). We noted no significant differences in germination rates between 5℃ and 10℃, and between 15℃ and 30℃. The highest positive slope of germination was noted at 25℃; however, it was reduced at 30℃ (Table 3). The fitted regression line to the germination rate evidenced a positive slope between 5℃ and 20℃ and a negative slope between 20℃ and 30℃. The intersection of fit linear regression between 5℃ and 20℃ and between 20℃ and 30℃ with the abscissa showed a T base of 1.76 and a T maximum of 33.10, respectively (Table 3 and Fig. 4). The intersection of the two linear regression lines of the germination rate at sub-optimal and supra-optimal revealed the optimal temperature (21.56) (Table 3 and Fig. 4)
Seed germination is a complex process that is profoundly affected by environmental factors, particularly cardinal temperature (minimum temperature, optimum, and maximum temperatures). Our results demonstrated that seed germination is influenced by temperature. Many authors have previously reported this in their studies (Phartyal et al. 2002, Zehtab-Salmasi 2006). A positive linear relationship was shown to exist between temperature and the germination rate from 5℃ to 20℃ and 25℃, and a negative linear relationship was detected from 20℃ to 25℃ and 25℃ to 30℃ (Figs. 1 and 2); Kamkar et al. (2006) reported identical results in a previous study of the germination rate responses of three millet species to temperature (ranging from 5 to 45℃ with 5℃ interval). Ghaedi et al. (2009) reported that the optimal temperature for the germination of
We believe that the seeds harvested from the Qom dune desert evidenced greater vigor than those harvested from the Fars dune desert, as the maximum germination rate in the seeds harvested from Qom dune was 96, and the maximum germination rate in the seeds from the Fars dune desert was 60. High-quality seeds can germinate under broader temperature ranges than low-quality seeds. The base temperatures of 0.62℃ and 1.76℃ for