Reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl radicals and superoxide anions can be formed by different metabolic processes in living organisms. Exogenous factors such as tobacco smoke, ionizing radiation and certain pollutants, and endogenous factors such as normal aerobic respiration, are generally involved in cellular processes such as mutagenesis, carcinogenesis and premature aging. ROS have the ability to react with a large variety of easily oxidisable cellular components, such as proteins, lipids and lipoproteins (Fridovich 1995). Furthermore, oxidation is one of the major causes of food deterioration, resulting in the destruction of fat-soluble vitamins and the development of off-colors and toxicants (Yang et al. 2000, Ukeda et al. 2002).
Commercial antioxidant supplements such as butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), α-tocopherol and propyl gallate have been used to reduce oxidative damages in the human body (Sherwin 1990, Gulcin et al. 2002). However, it is suspected that these synthetic antioxidants are responsible for a number of side-effects such as liver damage and carcinogenesis (Lindenschmidt et al. 1986). As a result, consumers have become more health conscious and investigators are increasingly seeking natural antioxidant alternatives for use in foods or medicinal materials.
Plants have the ability to absorb the sun’s radiation and generate high levels of oxygen as a byproduct of photosynthesis. Oxygen is easily activated by ultra violet (UV) radiation and heat from sunlight to produce toxic ROS. Consequently, plants have developed the ability to produce various antioxidative compounds in order to protect them from the harmful effects of ROS (Aruoma 1998). Although there is an abundance of scientific literature on macroalgae antioxidant effects, less comparative attention has historically been afforded to microalgae because of difficulties in their isolation and cultivation. However, the handful number of studies carried out to date have yielded promising results. For example, the antioxidative activity of phycocyanobilin from Spirulina platensis was evaluated against the oxidation of methyl linoleate in a hydrophobic system or with phosphatidylcholine liposomes (Hirata et al. 2000). Phycocyanobilin effectively inhibited the peroxidation of methyl linoleate and produced a prolonged induction period. Other microalgae such as blue-green algae and flagellates (Mynderse et al. 1977, Murakami et al. 1982) have also been reported to possess pharmacologically active compounds (Furukawa et al. 1993, Rho et al. 1995). Commercially, microalgae such as Chlorella sp., Spirulina sp. and Dunaliella sp. are grown for the production of algal products such as β-carotene, lutein and phycocyanin. But increasingly, microalgae are being investigated for properties beneficial to the nutraceutical and health foods industries.
The aim of this study was to assess the antioxidant potential of
1,1-Diphenyl-2-picrylhydrazyl (DPPH), thiobarbituric acid (TBA), trichloroacetic acid (TCA), sodium nitroprusside, sulphanilic acid, naphthylethylenediamine dihydrochloride, xanthine, xanthine oxidase from butter milk, nitro blue tetrazolium salt (NBT), BHT, α-tocopherol, 3-(2-Pyridyl)-5,6-di (p-sulfophenyl)-1,2,4-triazine disodium salt (ferrozine), potassium ferricyanide (K3Fe(CN)6 ), Folin-Ciocalteu reagent and linoleic acid were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). 2, 2-Azino-bis (3-ethylbenz-thiazolin)-6-sulfonic acid (ABTS), peroxidase and 2-deoxyribose were purchased from Fluka Chemie (Buchs, Werdenberg, Switzerland). Food grade digestive enzymes Viscozyme, Celluclast, AMG, Termamyl, Ultraflo, Protamex, Kojizyme, Neutrase, Flavourzyme and Alcalase were purchased form Novo Co. (Novozyme Nordisk; Bagsvaerd, Copenhagen, Denmark). All the other chemicals used were of analytical grades.
>
Sample collection and identification
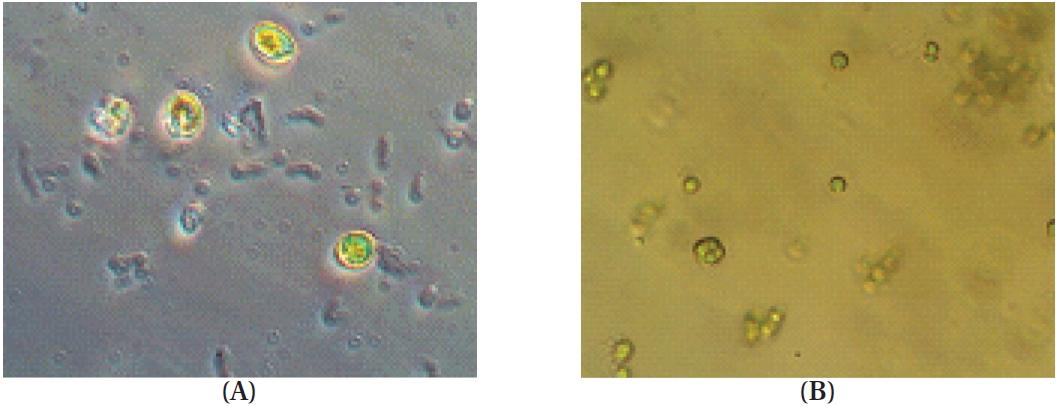
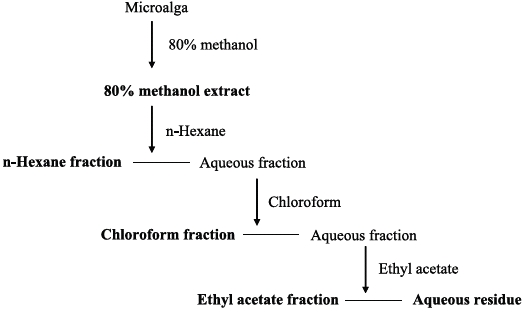
Phytoplankton samples were collected from coastal tidal pools in Gangjung village, Jeju Island, Korea. Specifically, the samples were collected from numerous small tidal pools located on top of volcanic rocks. Environmental factors such as water temperature, pH and salinity were measured at each sampling spot before the specimens were put in a flask containing F/2 nutrients media (Aquacenter Inc., Leland, MS, USA). The flasks were then immediately transferred into a plant growth chamber (VS-3D; Vision Scientific, Bucheon, Korea) and incubated for a week. In order to establish the most favorable condition for growth, samples were kept under a 12L : 12D cycle and subjected to temperatures of 15, 20, 25, and 30℃, with salinity of 25, 30, and 35 psu, respectively. The incubated samples were monitored periodically for pH and salinity. Phytoplankton samples were observed under a phase-contrast microscope for isolation and identification (Fig. 1). Identification was carried out based on the monograph of Tomas (1996).
Mass culture for each algal strain was carried out using artificial seawater media, which was prepared with F/2 nutrients media and trace element solution. Mass culture for phytoplankton was carried out in 10 liter media carrying capacity bottles (Transparent Polycarbonate Containers, Nalgene, Pittsburgh, PA, USA) with autoclaved artificial seawater media, which was enriched with F/2 nutrients media (Aquacenter Inc.). The salinity, temperature, pH, L : D cycle and light intensity of the culture were 35 psu, 20℃, 8.20, 12 : 12 and 180 μE m-2 s-1, respectively for
>
Preparation of 80% methanol extract and solvent fractions
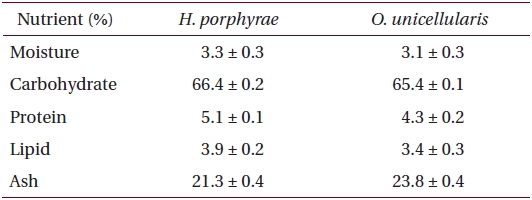
Freeze-dried samples were ground into a fine powder, after which 5 g samples were extracted with 80% methanol (500 mL) for 24 h at 25℃. The mixture was filtered and the 80% methanol extract was collected and concentrated. The extracts were obtained in sequence fractionation with a separatory funnel using three organic solvents (
>
Preparation of Enzymatic digests
Freeze-dried samples were ground into a fine powder, after which one gram was mixed with 100 mL of distilled water. The optimum pH of each reaction mixtures was adjusted with 1 M HCl / NaOH. Optimum pH and temperature conditions for the respective enzymes were as described by Heo et al. (2003). For this investigation, food grade enzymes consisting of five carbohydrases (Viscozyme, Celluclast, AMG, Termamyl and Ultraflo) and five proteases (Protamex, Kojizyme, Neutrase, Flavourzyme and Alcalase) were used. Enzymes were added to the sample at the ratio of 1% and incubated for 24 h. Subsequently, the reaction mixture was filtered and the enzyme was inactivated by heat (100°C for 10 min). Finally, the pH of each hydrolysate was adjusted to 7 with 1 M HCl / NaOH.
Proximate chemical composition of freeze-dried samples was determined according to the AOAC methods (Cunniff 1995). Crude lipid content was determined by the Soxhlet method, while crude protein content was determined by the Kjeldhal method. Ash content was determined by calcinations in furnace at 550°C while moisture content was determined by weighing the sample prior to and after 24 h in a dry oven at 105°C. Crude protein content in fractions and extracts was determined by the Lowry method, while the polysaccharide content was determined by the phenol-sulfuric method.
>
DPPH free radical scavenging assay
Free radical scavenging activity of the samples was determined according to the modified methodology of Brand-Williams et al. (1995). Each sample (2 mL) was mixed thoroughly with 2 mL of freshly prepared DPPH solution (3 × 10-5 M), after which the reaction mixture was incubated at room temperature for 30 min and its absorbance recorded at 517 nm using a UV?VIS spectrophotometer (Opron 3000; Hanson Tech. Co., Seoul, Korea).
>
Hydrogen peroxide scavenging assay
The ability of samples to scavenge H2O2 was determined according to the methodology of Muller (1985). Each sample (80 μL) and 20 μL of 10 mM hydrogen peroxide were mixed with 100 μL of phosphate buffer (0.1 M, pH 5.0) in a 96-microwell plate and incubated at 37℃C for 5 min. Thereafter, 30 μL of freshly prepared 1.25 mM ABTS and 30 μL of peroxidase (1 U mL-1) were mixed and incubated at 37℃ for 10 min, after which absorbance was measured at 405 nm.
>
Superoxide anion scavenging assay
Measurement of superoxide anion scavenging activity of the samples was based on the methodology of Nagai et al. (2003). A mixture of 0.48 mL of 0.05 M sodium carbonate buffer (pH 10.5), 0.02 mL of 3 mM xanthine, 0.02 mL of 3 mM ethylenediaminetetraacetic acid (EDTA), 0.02 mL of 0.15% bovine serum albumin, 0.02 mL of 0.75 mM NBT and 0.02 mL of sample was incubated at 25℃ for 10 min. The reaction was then started by adding 6 mU XOD and kept at 25℃ for 20 min. The reaction was stopped by adding 0.02 mL of 6 mM CuCl. Absorbance was measured in a microplate reader (Sunrise; Tecan Co. Ltd., Salzburg, Austria) at 560 nm.
>
Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity was determined according to the methodology of Chung et al. (1977). The Fenton reaction mixture (200 μL of 10 mM FeSO4.7H2O, 200 μL of 10 mM EDTA and 200 μL of 10 mM 2-deoxyribose) was mixed with 1.2 mL of 0.1 M phosphate buffer (pH 7.4), and with 200 μL of the sample. Subsequently, 200 μL of 10 mM H2O2 was added and incubated at 37℃ for 4 h, after which 1 mL of 2.8% TCA and 1 mL of 1% TBA were mixed and placed in a boiling water bath (10 min). After cooling, the mixture was centrifuged (5 min, 395 × g) and absorbance was measured at 532 nm.
>
Nitric oxide radical scavenging assay
Nitric oxide radical scavenging was determined according to the methodology of Garrat (1964). Two milliliter of 10 mM sodium nitroprusside in 0.5 mL of phosphate buffer saline (pH 7.4) was mixed with 0.5 mL of each sample and incubated at 25℃ for 150 min. From the incubated mixture, a 0.5 mL aliquot was removed and added in to 1.0 mL sulphanilic acid reagent (0.33% in 20% glacial acetic acid), then incubated at a room temperature for 5 min. Finally, 1.0 mL naphthylethylenediamine dihydrochloride (0.1% w/v) was mixed and incubated at room temperature for 30 min and absorbance was measured at 540 nm.
>
Ferrous ion chelating effect
The chelating of ferrous ions was estimated according to the methodology of Decker and Welch (1990). Each sample (5 mL) was added to a 0.1 mL of 2 mM FeCl2. The reaction was started by adding 0.2 mL of 5 mM ferrozine solution and subsequently incubated for 10 min at room temperature in a shaking incubator. After incubation, absorbance of the reaction mixture was measured at 562 nm.
>
Determination of lipid peroxidation inhibitory effect with the ferric thiocyanate (FTC) method
FTC method was conducted as described by Kikuzaki and Nakatani (1993). Each two milliliter sample (100 mg L-1) was mixed with 2 mL of 2.51% linoleic acid in ethanol, 4 mL of 0.05 M of phosphate buffer (pH 7) and 2 mL of distilled water, and the kept at 40℃ in a darkroom. A total 0.1 mL of the above mixture was added to 9.7 mL of 75% ethanol and 0.1 mL of 30% ammonium thiocyanate, and after 5 min 0.1 mL of 0.02 M ferrous chloride in 3.5% HCl was also added. Absorbance was measured every 24 h for 7 days.
Total phenolic compounds in the extracts were determined with Folin?Ciocalteu reagent according to the methodology of Chandler and Dodds (1993), using gallic acid as a standard phenolic compound. Each sample (1 mL) was mixed with 1 mL of 95% ethanol, 5 mL of distilled water and 0.5 mL of 50% Folin-Ciocalteu reagent. The mixture was allowed to react for 5 min after which 1 mL of 5% Na2CO3 was added and thoroughly mixed. The mixture was then placed in a darkroom for 1 h before absorbance was measured at 725 nm.
Statistical analyses were conducted with the SPSS (Version 11.5, SPSS Inc., Chicago, IL, USA) software package using triplicate (n = 3) test data. Mean values of each treatment were compared using one-way analysis of variance followed by Turkeys tests. A p-value of less than 0.05 was considered significant.
The temperature, pH and salinity of the tidal pools in Jeju Island varied from 12.7 to 27.5℃, 6.59 to 8.22, and 17.4 to 35.4 psu, respectively. Mono-strain samples of
[Table 1.] Proximate composition of Halochlorococcum porphyrae and Oltamannsiellopsis unicellularis
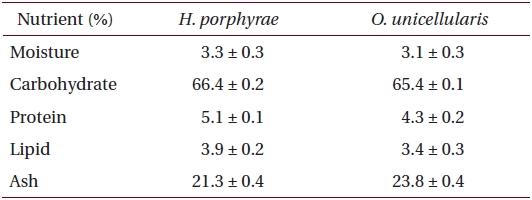
Proximate composition of Halochlorococcum porphyrae and Oltamannsiellopsis unicellularis
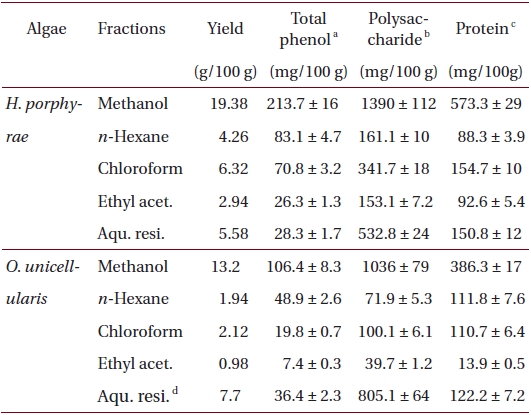
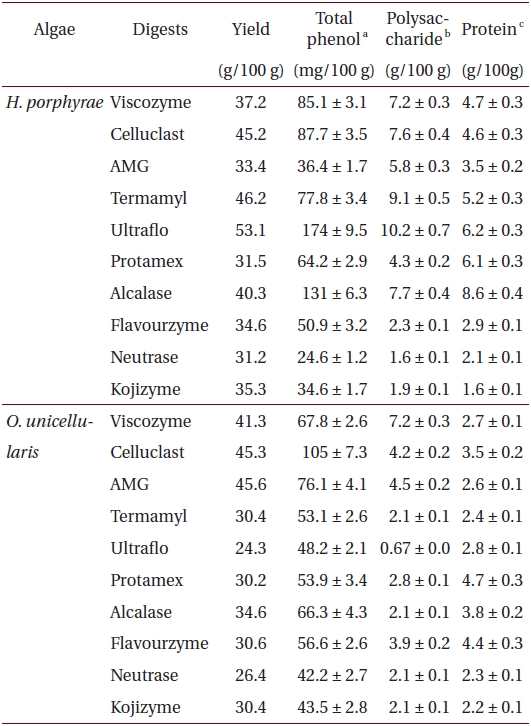
Total phenolic, polysaccharide and protein contents of 80% methanol extract, and its different fractions from Halochlorococcum porphyrae and Oltamannsiellopsis unicellularis
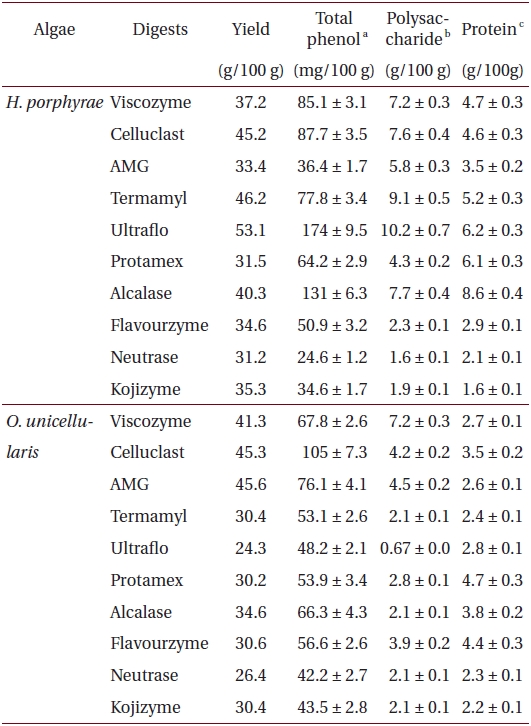
Total phenolic, polysaccharide and protein content of different enzymatic digests from Halochlorococcum porphyrae and Oltamannsiellopsis unicellularis
The proximate composition of freeze dried
>
DPPH free radical scavenging effects
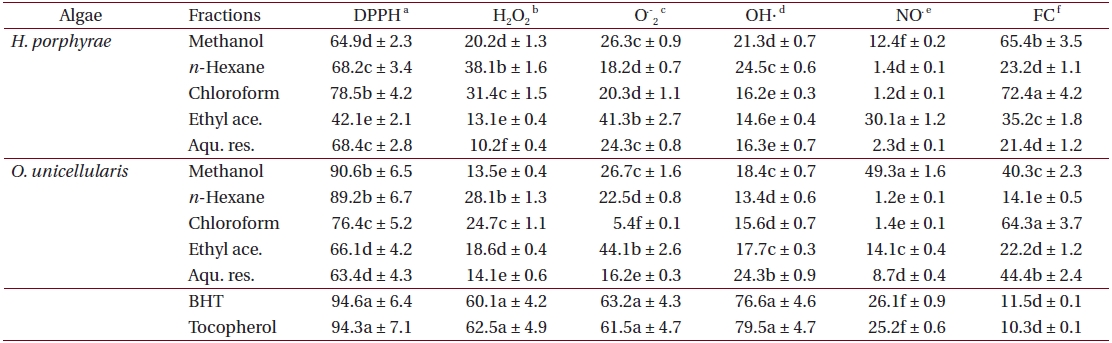
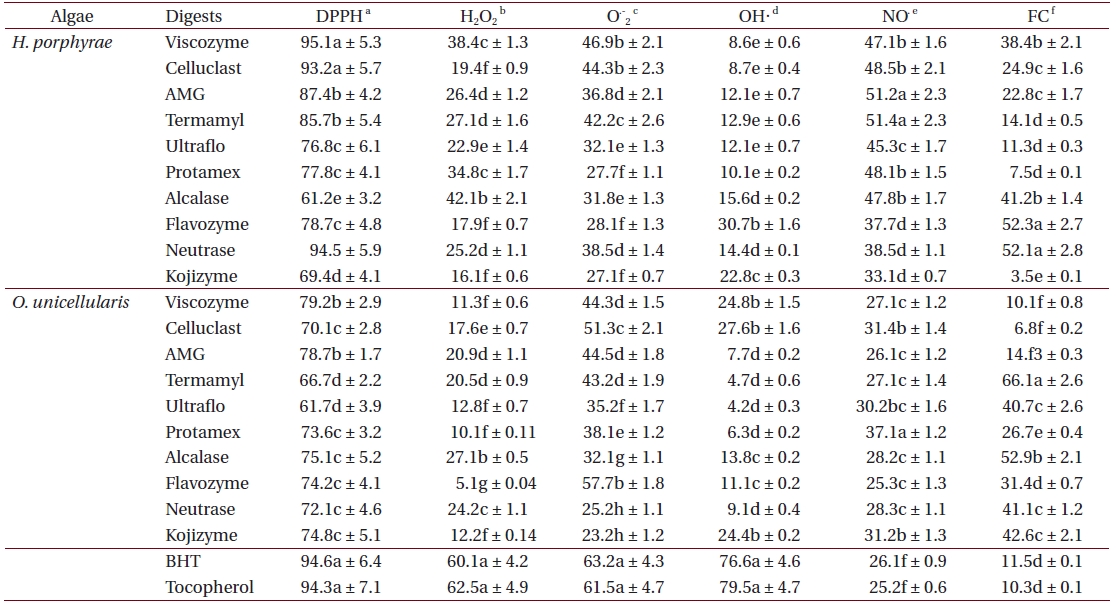
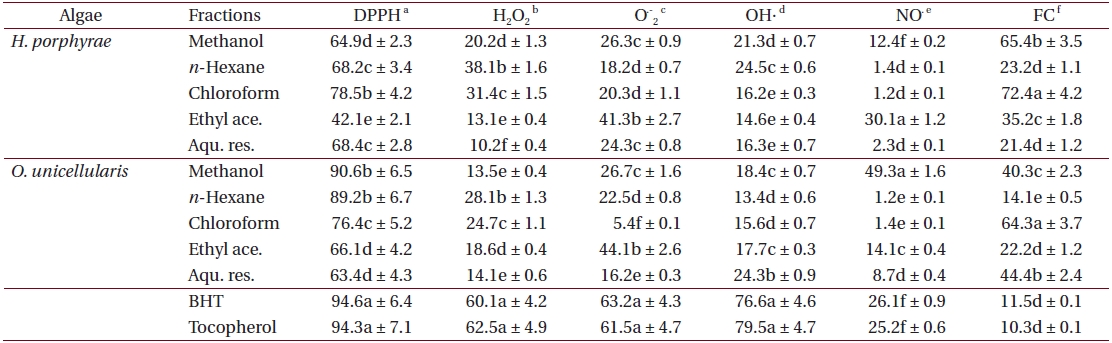
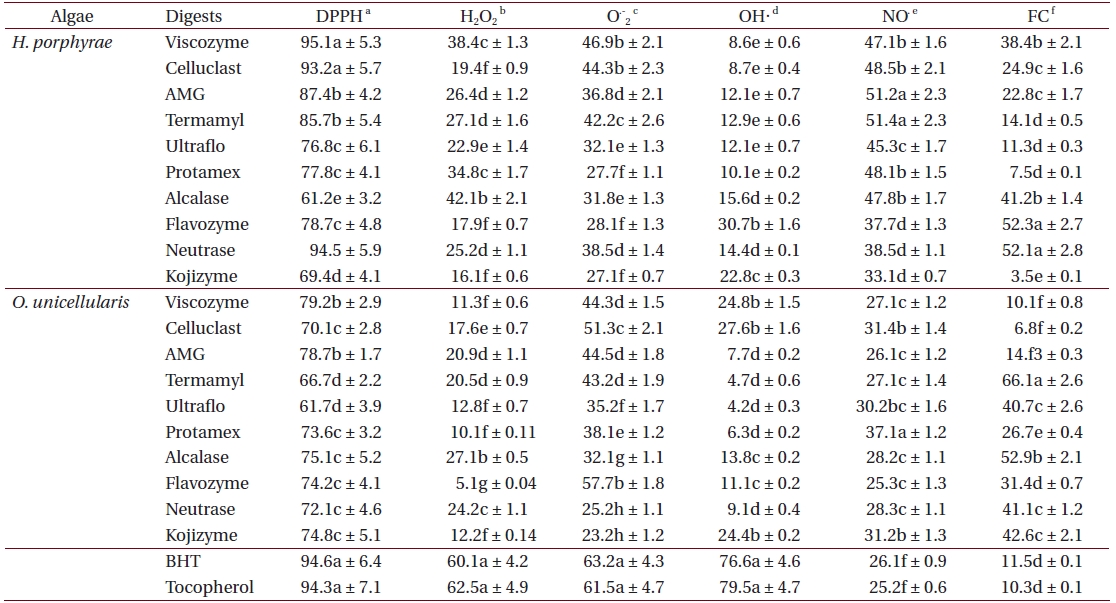
As shown in Table 4, the chloroform fraction of H. porphyrae exhibited the highest DPPH free radical scavenging activity (78.5%), followed by the n-hexane fraction and the aqueous residue (68.2% and 68.4%, respectively). In O. unicellularis, the 80% methanol extract exhibited the highest DPPH free radical scavenging activity (90.6%), followed by the n-hexane fraction and the chloroform fraction (89.2% and 76.4 %, respectively). It is notable that free radical scavenging activity decreased significantly (p < 0.05) from the 80% methanol extract towards the final aqueous fraction. According to Table 5, H. porphyrae, Viscozyme (95.1%) and Neutrase digest (94.5%) exhibited the highest DPPH free radical scavenging effects, followed by Celluclast (93.2%) and AMG digest (87.4%). In addition, Termamyl (85.7%), Flavozyme (78.7%), and Protamex digest (77.8%) showed considerable effects in DPPH free radical scavenging. In O. unicellularis, enzymatic digests by Viscozyme (79.2%), AMG (78.7%), Alcalase (75.1%), Kojizyme (74.8%), and Flavozyme (74.2%) exhibited strong DPPH free radical scavenging effects (Table 5). In addition, the Protamex, Celluclast and Termamyl digests demonstrated activities as 73.6%, 70.1% and 66.7%, respectively.
Antioxidant activity of 80% methanol extract and solvent fractions from Halochlorococcum porphyrae and Oltamannsiellopsis unicellularis
Antioxidant activity of enzymatic digests from Halochlorococcum porphyrae and Oltamannsiellopsis unicellularis
>
Hydrogen peroxide scavenging effects
Fractions of
>
Superoxide anion scavenging effects
The ethyl acetate fraction of
>
Hydroxyl radical scavenging effects
The
>
Nitric oxide radical scavenging effects
Among all the solvent fractions, the ethyl acetate of H. porphyrae (30.1%) and the 80% methanol extract (49.3%) of O. unicellularis exhibited significantly higher (p < 0.05) nitric oxide radical scavenging effects than those of the commercial antioxidants (Table 4). However, the remaining fractions exhibited fewer nitric oxide scavenging effects. According to Table 5, unlike organic solvent fractions from the 80% methanol extract, all the enzymatic digests indicated significant nitric oxide radical scavenging effects (p < 0.05) comparing to the commercial antioxidants. Of the H. porphyrae digests, Termamyl (51.4%), AMG (51.2%), Celluclast (48.5%), Protamex (48.1%), Viscozyme (47.1%), and Alcalase (47.8%) proved more significant (Table 5) (p < 0.05). Enzymatic digests of Protamex (37.1%), Celluclast (31.4%), and Ultraflo (30.2%) from O. unicellularis showed significant effects (p < 0.05).
>
Ferrous ion chelating effects
The chloroform fraction (72.4%), the 80% methanol extract (65.4%) and the ethyl acetate fraction (35.2%) from
>
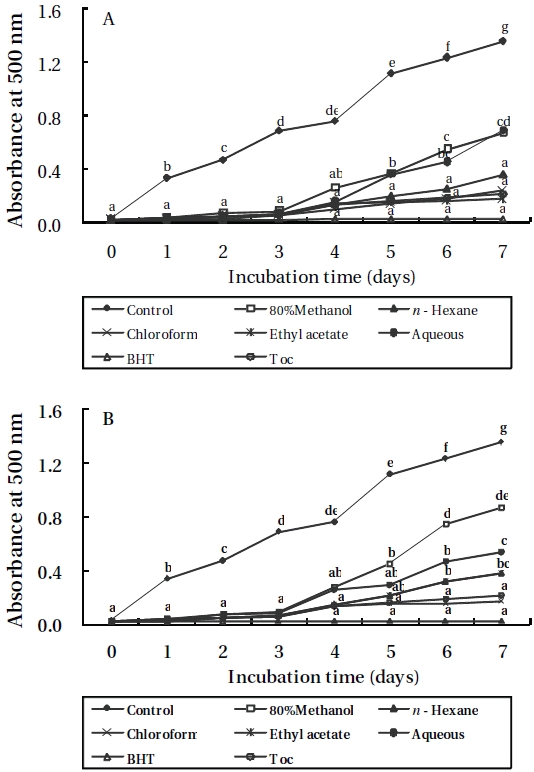
Lipid peroxidation inhibitory effects
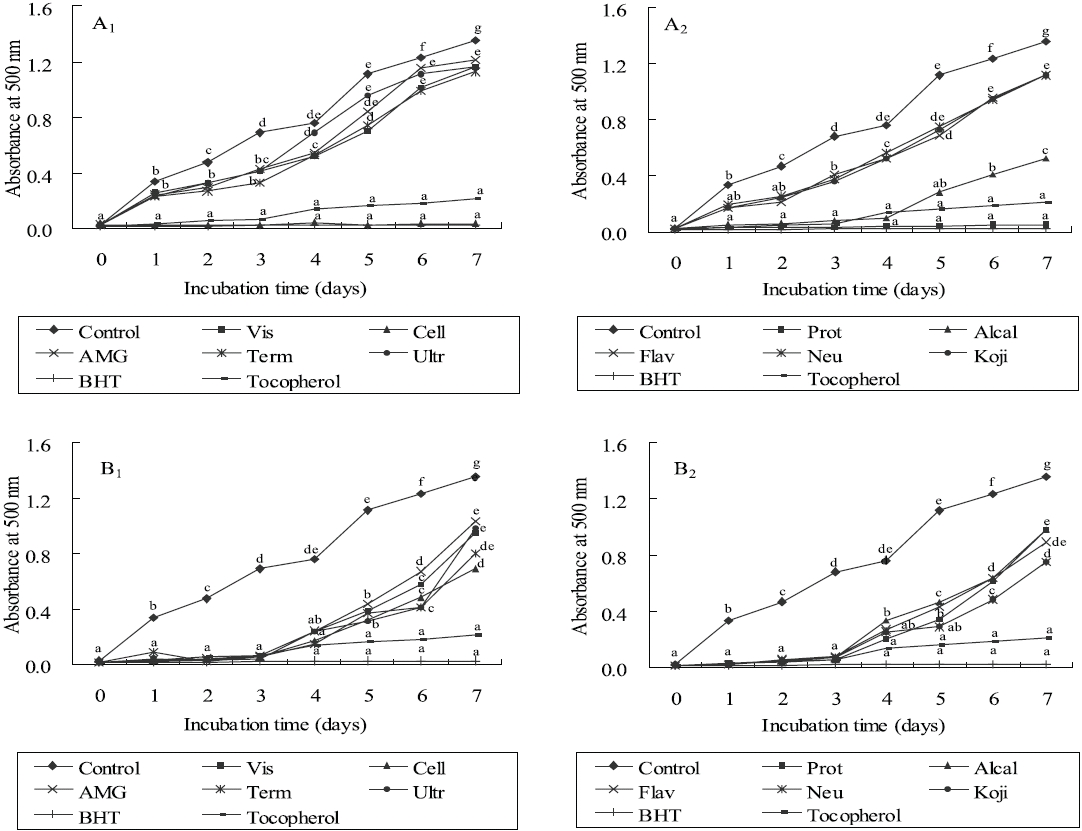
As shown in Figures 3 and 4, the absorbance of linoleic acid emulsion increased without the addition of
Small tidal pools are usually subjected to tidal interactions and as such exhibit different environmental and physical conditions to those of the sea. Specifically, their conditions are affected by submersion and exposure, rainfall, and evaporation, which are ultimately responsible for the creation of oligohaline (0.5 psu) to hyperhaline (above 80 psu) conditions. Such extreme environmental conditions are usually only tolerable by a handful number of phytoplankton species, in this case mono-strains of
Since these plants are especially susceptible to environmental stresses, they contain very potent antioxidants such as polyphenolics, carbohydrates, and nitrogen containing compounds, phytosterols, carotonoids and chlorophyll derivatives.
The effect of antioxidants on DPPH free radical scavenging was considered to be due to their hydrogen donating ability. In this study, the 80% methanol extract and organic solvent fractions of both algae showed notable activities indicating the higher efficacy for scavenging of free radicals. Furthermore, these results demonstrated that free radical scavenging compounds had both hydrophilic and hydrophobic properties. In addition, it is obvious that the enzymatic digests also possessed strong DPPH free radical scavenging effects. Thus, both carbohydrases and proteases have the capability to liberate bioactive compounds for radical scavenging. The implications are important as radical scavengers may protect cell tissues from free radicals, thereby preventing diseases such as cancer (Nakamura et al. 1996).
According to our results, it was apparent which bioactive compounds cause the greatest H2O2 scavenging disperses in solvent fractions and enzymatic digests, thus showing both hydrophilic and hydrophobic characteristics. In addition, it could be seen that enzymatic hydrolysis is also effective in releasing compounds that are responsible for H2O2 scavenging. However, a small effect can be observed on the scavenging of H2O2 in this study. H2O2, a reactive non-radical compound, is very important as it can penetrate biological membranes. Thus, removing H2O2 is critical for the protection of living systems. The addition of H2O2 to cells in culture can lead to transition of metal ion-dependent OH. mediated oxidative DNA damage (Halliwell 1991).
Higher superoxide anion effect was observed in the ethyl acetate fraction in both algae. Furthermore, results from the enzymatic digests exhibited considerable effects, particularly in O. unicellularis where the scavenging activity of enzymatic digests had moderate activities, and those activities were mainly concentrated in the carbohydrase digests. Therefore, it was obvious that the superoxide anion scavenging compounds present in solvent fractions and enzymatic digests had both hydrophilic and hydrophobic properties. A superoxide anion radical generally forms first and its effects can be exaggerated as it produces other kinds of cell damage inducing free radicals and oxidizing agents (Liu and Ng 2000). It is a precursor to active free radicals that have a potential to react with biological macromolecules and thereby induce tissue damage (Halliwell and Gutteridge 1989).
The hydroxyl radical scavenging activity of enzymatic digests and solvent fractions from both algae was determined as the percentage of inhibition of hydroxyl radicals generated in the Fenton reaction mixture. In this study, it was noticeable that the hydroxyl radical scavenging compounds exhibited more hydrophilic properties according to the solvent fractionation results. In addition, except for the Flavozyme digest from H. porphyrae, other enzymatic digests did not exhibit satisfying effects in hydroxyl radical scavenging. Hydroxyl radical is the most ROS among all ROS due to its strong ability to react with various biomolecules. The hydroxyl radical reacts oxidatively with several biological materials through hydrogen withdrawal, double bond addition, electron transfer, and radical formation, and initiates autoxidation, polymerization, and fragmentation (Liu and Ng 2000).
Nitric oxide is a gaseous free radical, which is of concern in cancer, inflammation, and other pathological conditions. In nitric oxide scavenging, the enzymatic digests showed higher activities than the solvent fractions, and those activities were significantly higher than the standard antioxidants. Therefore, it can be suggested that those bioactive compounds were relatively hydrophilic. In addition, enzymatic hydrolysis was effective in extracting biochemical compounds that were responsible for NO? inhibition. The activities of NO? and O2-? were found to be relatively low, but their metabolites ONOO- (peroxynitrite) are extremely reactive and directly induce toxic reactions, including SH-group oxidation, protein tyrosine nitration, lipid peroxidation and DNA modifications (Moncada et al. 1991, Radi et al. 1991).
Ferrozine can make red colored complexes with ferrous ions. In the presence of chelating agents, this complex formation is interrupted and as a result, the red color of the complex decreases. Thus, the chelating effect of the coexisting chelator can be determined by measuring the rate of color reduction. In this study, the formation of the ferrozine-Fe2+ complex is interrupted in the presence of algae extracts, indicating significant chelating ability. Both the organic solvent fractions and the enzymatic digests had significant metal chelating effects (p < 0.05). It was apparent that bioactive compounds which cause metal chelating have hydrophilic and hydrophobic properties. Ferrous can initiate lipid peroxidation by a Fenton reaction as well as accelerate peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals (Halliweill 1991, Fridovich 1995). Thus, H. porphyrae and O. unicellularis demonstrated a marked capacity for iron binding, suggesting their ability as peroxidation protectors, which indicate iron binding capacity (Gulcin et al. 2004).
To evaluate the antioxidant effects of H. porphyrae extracts, their lipid peroxidation inhibition was compared with commercial antioxidants by determining the amount of peroxide formed in emulsion during the incubation period. High absorbance is an indication of high concentration of formed peroxides. In this experiment, the ethyl acetate fraction, the enzymatic digests by Celluclast, and Protamex from H. porphyrae, as well as the chloroform fraction of O. unicellularis provided the highest lipid peroxidation inhibition among all the solvent fractions and enzymatic digests. This showed that those extracts were able to reduce the formation of peroxides. It has been suggested that different antioxidant components were liberated from the inside of microalgae cells because of enzyme digestion. These extracts contained high levels of polysaccharides, proteins and polyphenols, which may lead to the assumption that those components may influence lipid peroxidation inhibition.
Previously it has been reported that antioxidant activity is correlated with polyphenolic contents (Lu and Foo 2000, Oki et al. 2002). In this study, some enzymatic digests did not possess antioxidant activity, although they contained numerous phenolic compounds. Therefore, it can be assumed that polyphenol content is not the only factor that can influence antioxidant activity. In fact, there are other bioactive components such as proteins, polysaccharides and different kinds of pigments which are present in microalgae (Kardosova and Machova 2006, Moure et al. 2006, Wang et al. 2007). For example it was established that oligosaccharides, sulfate and glycoprotein components have exhibited antioxidant activities in the red microalga, Porphyridium sp. (Spitz et al. 2005).
In this study, bioactive compounds extraction was performed using 80% methanol and different kinds of food grade enzymes to determine the antioxidant effects in Jeju tidal pool microalgae. As a solvent, methanol is extensively used in experiments for the extraction of potential bioactive compounds with hydrophilic and hydrophobic properties. By solvent fractionation, biological compounds may be distributed according to their polarity, resulting in different activities. However, plant cells originally contain large amounts of soluble polysaccharides and insoluble fibers such as cellulose. These fibers together with other cell wall materials act as a physical barrier for the extraction of some bioactive materials. To overcome those barriers, enzymatic hydrolysis is effective in the extraction of biochemical components. Enzymatic hydrolysis of raw material tissues or cells has resulted in significant yields of desired compounds and convenient industrial approaches in extraction and purification (Heo et al. 2003, Siriwardhana et al. 2004). In addition, the breakdown and releasing of high molecular weight polysaccharides and proteins themselves may contribute to enhance the antioxidative activities (Penta-Ramos and Xiong, 2002, Ruperez et al. 2002). Of the several advantages of enzymatic extraction, water solubility, higher extraction efficacy, a greater variation of constituents, minimized environmental pollution and comparative inexpensiveness is obvious.
In conclusion, the results of this study have highlighted potential antioxidant activity against ROS, especially in enzymatic digests. As such, the use of H. porphyrae and O. unicellularis as natural antioxidant sources in the food and pharmaceutical industries appears promising and should be investigated further.