Although seagrass beds only cover 0.1-0.2% of global coastal habitats, they represent a highly productive ecosystem which plays an important role in the health of local biota (Duarte 2002). However, because seagrass beds are restricted to coastal habitats they are subject to pressures from human activities, and thus significant declines of seagrass coverage have been documented worldwide (Spalding et al. 2003, Kenworthy et al. 2006). Transplantation of seagrasses has been conducted as a remedial conservation or management tool (Davis and Short 1997, Orth et al. 1999, Fishman et al. 2004, Paling et al. 2007, Park and Lee 2007). More recently, transplantation trials on the most widely distributed seagrass species in Korea,
Plants often exhibit a remarkable capacity to adjust their morphology and physiology to the local environmental conditions (Sultan 2000, Pigliucci 2001, Callaway et al. 2003). Seagrasses also exhibit substantial plasticity in morphology and growth, which is considered as an important component of their capacity to adapt to disturbances, resource heterogeneity and other environmental variations (Guidetti 2000, Hemminga and Duarte 2000, van Katwijk et al. 2000, Rhode and Duffy 2004). Seasonal variations in seagrass shoot morphology, biomass and growth in response to changing environmental conditions are an obvious example of phenotypic plasticity (Duarte 1989, Olesen and Sand-Jensen 1994, Laugier et al. 1999). The extent of intraspecific variability is believed to reflect their capacity and strategy to colonize and survive from environmental changes (Marba and Duarte 2003). When seagrass transplantation is performed, transplants that survive and adapt to the new environmental conditions of the transplant site may exhibit newly observed phenotypic changes (van Tussenbroek 1996, van Katwijk et al. 2000).
Although Z. marina transplantation has previously been attempted in Korea, few studies have closely examined the adaptive characteristics of the transplants in their new environment. In this study we examine the characteristics of a transplanted seagrass bed in Jindong Bay (Fig. 1), which is located on the southern coast of Korea (35°05.6' N, 128°33.9' E). The Jindong Bay transplantation exercise was conducted in 2004 to help restore lost seagrass meadows which had disappeared due to anthropogenic activities. Z. marina shoots for transplantation were collected from Koje Bay (34°48.0'N, 128°35.0'E) located approximately 33 km south of Jindong Bay. Our investigation began three and a half years following the transplantation in July 2008, where for the next 12 months shoot morphology, growth, and reproduction strategy of Z. marina were compared between the donor and transplant sites. We hypothesized that Z. marina plants will exhibit phenotypic variations in shoot morphology, growth, and reproduction strategy due to different environmental conditions between the two sites.
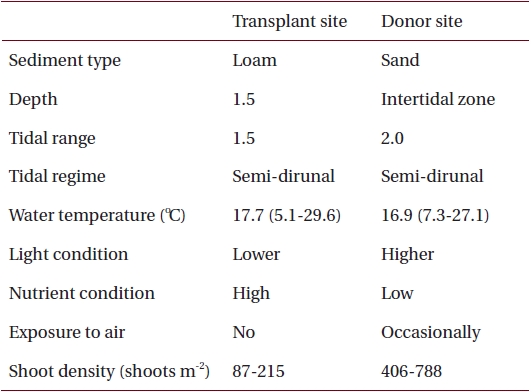
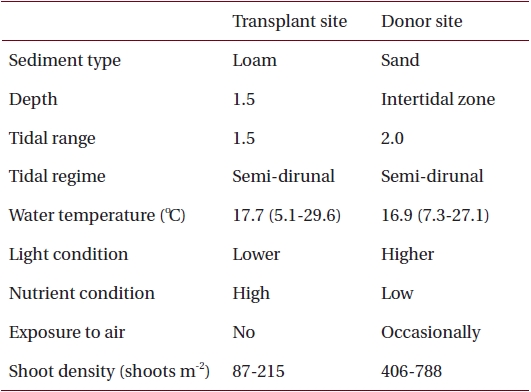
Environmental characteristics at the transplant and donor sites on the southern coast of Korea
Sediments at the transplant site were characterized by loam, the tidal regime was semi-diurnal with a 1.5-m tidal range and water depth was about 1.5 m below mean sea level (Table 1, Fig. 2). Sediments at the donor site were characterized by high sand content, the tidal regime was semi-diurnal with a 2.0-m tidal range, and the donor bed was located in the intertidal zone (Table 1, Fig. 2).
Water temperature at the seagrass canopy level in the transplant and donor sites were monitored continuously using HOBO data loggers (Onset Computer Corp., Bourne, MA, USA) encased in a waterproof underwater housing every 15 min from July 2008 to June 2009. Measured water temperature was averaged daily.
Transplantation of
Shoot morphology, individual shoot weight, leaf productivity and lateral shoot production of
For leaf productivity measurements, the leaf marking method was used according to Short and Duarte (2001). At each sampling event, 12 to 14 seagrass shoots were pierced through the bundle sheath about 3 cm above basal meristem using a hypodermic needle. Marked plants were retrieved 4-5 weeks after marking and all the leaf tissues were separated into old (produced before piercing) and new (produced after piercing) leaf tissues. The separated leaf tissues were dried at 60<℃ to a constant weight. Leaf productivity (mg DW shoot-1 d-1) was calculated by dividing the dry weight of the new leaf tissues of each shoot by the time interval between the marking and the retrieval (i.e., days).
Data are reported as mean ± standard error. Statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Data were tested for normality and homogeneity of variance to meet the assumptions of parametric statistical analysis. If these assumptions were not satisfied, data were log transformed. Differences in water temperature, shoot morphology, individual shoot weight, leaf productivity and lateral shoot production among sampling times and between study sites were tested for significance using a two-way ANOVA and a post-hoc analysis. A p-value of less than 0.05 was considered significant.
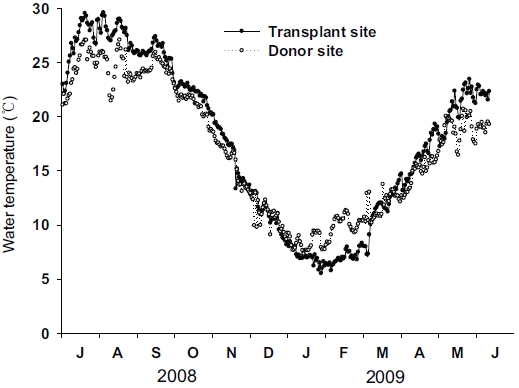
Distinct seasonal variations in water temperature were found in both the transplant and donor sites (Fig. 3). Water temperature was highest in July 2008 and lowest in January 2009 at both sites. Although seasonal variations in water temperature in the two sites were synchronous, the variation amplitude was slightly different between the two sites. The highest water temperature at the transplant site (29.6℃) was slightly higher than that at the donor site (27.1℃), while the lowest water temperature was slightly lower at the transplant site (5.5℃) than at the donor site (7.3℃).
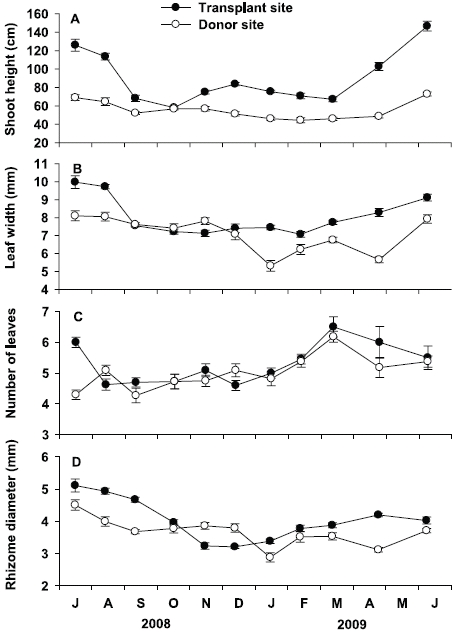
Shoot height at the transplant site was significantly (p < 0.001) higher than that at the donor site throughout the study period (Fig. 4A). Seasonal variation of shoot height at the transplant site was statistically significant (p < 0.001), with lowest values in October 2008 (58.1 ± 1.7 cm) and highest in June 2009 (146.6 ± 5.4 cm). Seasonal variation of shoot height at the donor site was also statistically significant (p < 0.001), but variation amplitude was much smaller than that at the transplant site. Shoot height was highest in June 2009 (72.8 ± 2.2 cm) and lowest in February 2009 (44.4 ± 2.7 cm) at the donor site. Leaf width at the transplant site was also significantly (p < 0.001) wider than that at the donor site during experimental period, except in September, October, and December of 2008 (Fig. 4B). Leaf width at the transplant site ranged from 7.1 ± 0.2 mm in November 2008 to 10.0 ± 0.4 mm in July 2008, showing a significant (p < 0.001) seasonal variation. Leaf width at the donor site also showed significant (p < 0.001) seasonal variation, with highest values in July 2008 (8.1 ± 0.3 mm), and lowest in January 2009 (5.3 ± 0.3 mm).
Number of leaves were not significantly (p = 0.160) different between the transplant and donor sites (Fig. 4C). At the transplant site, the average number of leaves per shoot varied from 4.5 ± 0.2 in December 2008 to 6.5 ± 0.5 in May 2009, showing significant (p < 0.001) seasonal variation. Number of leaves per shoot at the donor site also showed significant (p < 0.001) seasonal variation, ranging from 4.3 ± 0.2 in September 2008 to 6.2 ± 0.2 in March 2009. Rhizome diameter at the transplant site was greater than that at the donor site during the study period, except in November and December 2008 (Fig. 4D). At the transplant site, rhizome diameter was highest in July 2008 (5.0 ± 0.1 mm), and lowest in December 2008 (3.2 ± 0.1 mm), showing significant seasonal variation (p < 0.001). Rhizome diameter at the donor site also showed significant seasonal variation (p < 0.001), with highest values in July 2008 (4.2 ± 0.2 mm), and lowest in January 2009 (2.8 ± 0.2 mm).
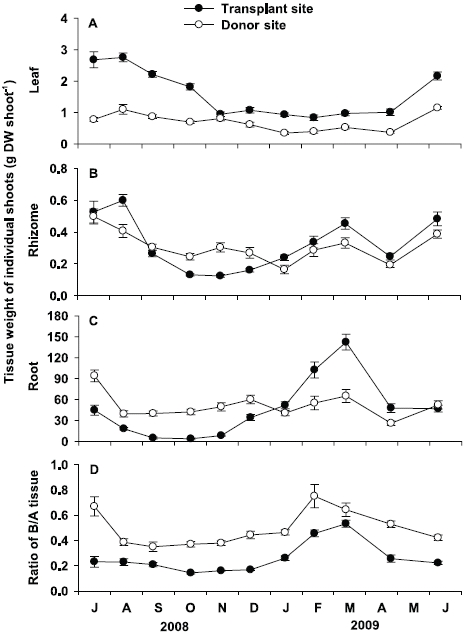
Leaf tissue weight of individual shoots at the transplant site was significantly (p < 0.001) higher than that at the donor site throughout the study period, except in November 2008 (Fig. 5A). At the transplant site, leaf tissue weight showed significant (p < 0.001) seasonal variation, with highest values in August 2008 (2.75 ± 0.14 g DW shoot-1) and lowest in February 2009 (0.84 ± 0.09 g DW shoot-1). At the donor site, leaf tissue weight of individual shoot also showed significant (p < 0.001) seasonal variation, with lowest values in January 2009 (0.35 ± 0.05 g DW shoot-1) and highest in June 2009 (1.15 ± 0.04 g DW shoot-1).
There were no significant (p = 0.808) differences in rhizome tissue weight of individual shoots between the two sites (Fig. 5B). At the transplant site, significant seasonal variation (p < 0.001) in rhizome tissue weight was observed, with highest values in August 2008 (0.60 ± 0.04 g DW shoot-1), and lowest in November 2008 (0.12 ± 0.01 g DW shoot-1). Significant seasonal variation (p < 0.001) in rhizome tissue weight was also found at the donor site, with highest values in July 2008 (0.50 ± 0.05 g DW shoot-1), and lowest in January 2009 (0.16 ± 0.03 g DW shoot-1, Fig. 5B).
No significant (p = 0.670) difference was also observed in root tissue weight of individual shoots between the two sites (Fig. 5C). However, seasonal variation in root tissue weight was more distinct at the transplant site than at the donor site. Root tissue weight of individual shoots at the transplant site ranged from 3.8 ± 1.2 mg DW shoot-1 in October 2008, to 142.3 ± 11.2 mg DW shoot-1 in March 2009. At the donor site, significant (p < 0.001) seasonal variation in root tissue weight was also observed, with lowest values in April 2009 (26.1 ± 3.3 mg DW shoot-1), and highest in July 2008 (94.1 ± 8.4 mg DW shoot-1).
Ratio of below- to aboveground tissue at the trans-plant site was significantly (p < 0.01) lower than the donor site (Fig. 5D). At the transplant site, significant (p < 0.001) seasonal variation in the below- to aboveground tissue weight was observed, ranging from 0.14 ± 0.01 in October 2008 to 0.53 ± 0.03 in March 2009. At the donor site, significant seasonal variations (p < 0.001) were also observed, with maximum values (0.75 ± 0.09) in February 2009 and minimum (0.35 ± 0.04) in September 2008.
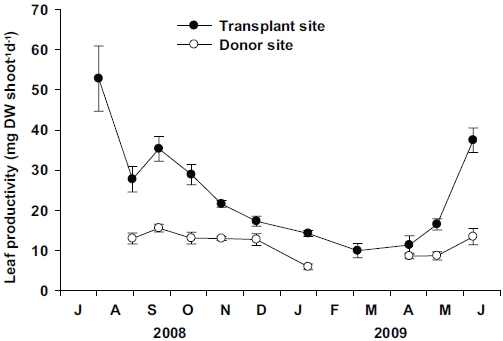
Leaf productivity per shoot at the transplant site was significantly higher (p < 0.01) than the donor site (Fig. 6). At the transplant site, significant seasonal variations (p < 0.001) in leaf productivity were observed, with the highest values (52.79 ± 8.12 mg DW shoot-1 d-1) in July 2008, and lowest (9.98 ± 1.77 mg DW shoot-1 d-1) in March 2009. Although some data were missing from the donor site, significant (p < 0.001) seasonal variations in leaf productivity were also observed, with the highest values (15.58 ± 0.96 mg DW shoot-1 d-1) in September 2008 and lowest (5.94 ± 0.72 mg DW shoot-1 d-1) in January 2009. More distinct seasonal variation in leaf productivity was observed at the transplant site than at the donor site due to the greater variations in individual shoot weight at the transplant site.
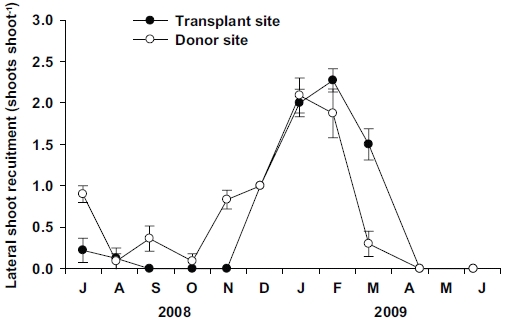
No significant differences (p = 0.814) were observed in the lateral shoot production between the transplant and donor sites (Fig. 7). However, vegetative reproduction strategy was different between the two sites. No lateral shoots were observed at the transplant site during September to November 2008, whereas a few lateral shoots were observed during this period at the donor site (Fig. 7). Clear seasonal variations (p < 0.001) in lateral shoot production were observed at both sites. Maximum values of lateral shoot production were observed in January 2009 at the donor site (2.1 ± 0.2 shoots shoot-1) and February 2009 at the donor site (2.3 ± 0.1 shoots shoot-1).
Plants possess a remarkable capacity to alter their phenotype in response to changes in environmental conditions (Sultan 2000, Pigliucci 2001, Callaway et al. 2003). Phenotypic plasticity is believed to be essential for the survival of plants in heterogeneous environments (Valladares et al. 2002). In this study, taller Z. marina shoot height was observed at the transplant site than at the donor site. Leaf width was also wider at the transplant site than at the donor site. These morphological changes may be correlated with changes in environmental conditions, such as light and nutrient conditions, air exposures, and wave action, etc. (den Hartog 1970, Bach 1993, Krause-Jensen et al. 2000, Peralta et al. 2000, Bostrom et al. 2004). Because changes in shoot and leaf size affect the capacity of light acquisition, light availability is an important factor in controlling seagrass morphological characteristics (Vermaat et al. 1996, Lee et al. 2004, Ralph et al. 2007). Seagrasses can respond to low light availability by changing their shoot and leaf morphology (Hemminga and Duarte 2000, Krause-Jensen et al. 2000, Olesen et al. 2002). Seagrass leaf and shoot tend to be longer in the deeper water as they extend towards the water surface for light capture (Dennison and Alberte 1986, Krause-Jensen et al. 2000, Bostrom et al. 2004, Krause-Jensen et al. 2004). In this study, water depth at the transplant site was deeper than the donor site. This disparity could have contributed to the larger shoot size and leaf length of Z. marina at the transplant site.
Furthermore,
Nutrient availability is another important factor that can affect seagrass shoot morphology (Moore and Short 2006). In this study, the donor site was characterized by high sand content sediments with low pore water nutrient content, whereas the transplant site had loamy sediments with high nutrient content (Table 1) (Park and Lee 2007). Therefore, it is possible that the lower sediment nutrient conditions at the donor site probably contributed to smaller Z. marina shoot size (Perez et al. 1994, Agawin et al. 1996). Shoot density is one of main factors determining the degree of resource competition between shoots (Gopal and Goel 1993, Hashemi et al. 2005). The higher shoot density at the donor site (Table 1) is probably responsible for nutrient limitation, which consequently contributes to the smaller shoot size in the area (Olesen and Sand-Jensen 1994, Meling-Lopez and Ibarra-Obando 1999, Marba and Duarte 2003). The lack of competition for resources appears to be why Z. marina shoots adapted well to the new transplant environment, and exhibited much higher shoot size than the donor site.
Rhizome diameter of seagrasses is considered to be a robust morphological characteristic which can be used as an indicator of plant status (Duarte 1991, Marba et al. 2006, Cabaco et al. 2008). It has been reported that shoot biomass of seagrass species can be calculated based on rhizome diameter (Marba et al. 2006). In this study, the larger rhizome diameter of seagrass shoots at the transplant site corresponded to larger shoot height and higher individual shoot weight than those at the donor site. Seagrass rhizomes with a larger diameter in the transplant site might allow the storage of large quantities of reserve carbohydrates, which will be mobilized to aboveground tissues during adverse environmental conditions or for new leaf growth (Lee and Dunton 1996, 1997, Vermaat 2009). Thus, increased rhizome diameter at the transplant site may be advantageous for survival and establishment of seagrass transplants in their new environments.
Leaf productivity was also significantly higher at the transplant site than at the donor site. Increased leaf productivity at the transplant site is probably attributable to high nutrient availability and low competition for nutrients. Higher leaf productivity per shoot at the transplant site can also be linked to the bigger shoot size due to lower shoot density at the site (Niklas and Enquist 2001, Ruiz and Romero 2003, Lee et al. 2005).
Nutrient conditions can affect seagrass biomass allocation as well as the seagrass growth (Lee and Dunton 1999). Seagrasses allocate more biomass in leaf tissues under high nutrient availability, but more belowground biomass under low nutrient conditions (Romero et al. 2006). Thus higher below- to aboveground tissue ratio will be observed in low nutrient conditions. In this study, the ratio of below- to aboveground tissue was significantly higher at the donor site than at the transplant site. The change in biomass allocation at the transplant site can be attributed to high nutrient availability in sediments.
Vegetative reproduction of seagrasses is achieved through rhizome branching, and thus small plants may produce a substantial number of new lateral shoots with a much lower cost than large ones (Duarte 1991). In Z. noltii, higher lateral shoot recruitment rates were associated with smaller shoot size (Laugier et al. 1999). In this study, vegetative reproduction through lateral shoot recruitment was achieved only in winter and spring at the transplant site, but was observed throughout all seasons (except late spring) at the donor site. No lateral shoot recruitment at the transplant site was observed from September to November 2008, during which minimum ratio of the below-/aboveground tissue occurred. The lack of lateral shoot recruitment during that period might be related to the decreasing rhizome weight at the transplant site. In conclusion, Z. marina shoots at the transplant site and the originally inhabited site exhibited phenotypic variations in shoot morphology, growth, and reproduction. Shoot size and growth increased at the transplant site due to the high nutrient availability and low physical stresses. Increased growth and shoot morphology of Z. marina at the transplant site imply that the transplants have adapted well and established successfully to their new environment.