The genus Rhodymenia Greville includes species with traditional used in human nutrition in Ireland and Brittany and more recently marketed as a health food (Le Gall et al. 2004). Rhodymenia palmata (L.) Kuntze contains as much as 35% proteins and is a rich source of vitamins and eicosapentanoic acid (Mishra et al. 1993) and was identified as one of the three red algal species with the best potential for seaweed cultivation in the northeastern United States and Canada (Cheney 1999). Due to its high protein content (Morgan et al. 1980; Fleurence 1999), Rhodymenia sp. was considered as a good food source for abalone aquaculture (Evans and Langdon 2000; Rosen et al. 2000). Rhodymenia pseudopalmata (Lamouroux) Silva is an important species of tidal and subtidal algal communities in the southern coasts of Argentina (Mendoza and Nizovoy 2000). Its fronds are flattened, fan-shaped, rather stiff, light brownish ? red fronds, with long or short stipes arising from a discoid base. Fronds are repeatedly dichotomously lobed, with axils wide, apices rounded and margins smooth.
This study is a part of a more general study in which we are focused on the identification of the most common epiphytes infecting the host R. pseudopalmata on southern coasts of Argentina. We found the occurrence of Laminarionema elsbetiae Kawai & Tokuyama. L. elsbetiae was first described on Laminaria japonica Areschoug from Japan (Kawai and Tokuyama 1995) and it is known in Europe only from Helgoland (Peters and Ellertsdottir 1996).
Algal species growing on or within other algae has been widely reported (Goff 1982; Ducker and Knox 1984; Correa et al. 1988, 1993; Correa 1990; Correa and McLachlan 1991, 1992, 1994). Are goals of the present paper to assess the kind the symbiosis interactions and the relative abundance including the estimation of the severity index of infection of L. elsbetiae on R. pseudopalmata. It was also a goal to study the biology of L. elsbetiae under both nature and culture conditions.
Rhodymenia pseudopalmata fronds were obtained from subtidal populations from the coast of Santa Isabel, (43°18’S-65°06’W) in the province of Chubut, Argentina during December, 2004. A collection of 30 randomly selected fronds was used for the present research.
Host thalli of R. pseudopalmata (Fig. 1) were divided in apical, intermediate and basal sections for their examination. Size, presence and position of individuals of L. elsbetiae and severity degree of infection were registered for each frond. In order to evaluate the severity degree of infection, a qualitative scale was used (Peters and Schaffelke 1996). This scale resulted from the visual categorization of a dissection observed by light microscopy. Prevalence (i.e., percentage of thalli that were infected) and severity of infection (i.e., mean abundance of L. elsbetiae on each host thallus) were then estimated. A ‘severity index’ was based on a semi-quantitative estimation of L. elsbetiae cover on host thallus using four categories, where 0 = a total absence of epiphytes, 1 = 1-30% cover, 2 = 31-70% cover, and 3 = 71-100% cover. The severity degree of infection was categorized as low when the percentage of host thalli colonized by L. elsbetiae ranged from 1% to 10% (i.e., no visible signs of endophytic infection were observed). The severity degree of infection was categorized as moderate when the percentage of colonized thalli varied from 11% to 70% (i.e., moderate alterations, such as brown spots on the lamina, were observed). Finally, the severity degree of infection was categorized as high in those cases in which thalli exhibited a colonized area percentage higher than 71% (i.e., strongly invaded thalli were observed). The distinction between the categories was arbitrary. Only those thalli under the categories ‘moderate’and ‘high’were considered diseased.
After being collected, fronds were kept on ice, retained in labeled plastic bags until they were examined in the laboratory, usually within 5 h after collection. Fronds were brushed and rinsed under running tap water. Small portions of infected fronds were sectioned, then immersed in fresh 0.5% solution of sodium hypochlorite for 30 s, and finally rinsed three times, 5 min each, in sterile seawater. A 2-min sonication was subsequently applied to 5 mm x 5 mm portions in sterile seawater, renewing the seawater after each burst. This cleaning procedure was followed in order to remove diatoms as well as other epiphytes.
L. elsbetiae crude cultures were initiated by inoculating portions of cleaned fronds in plastic Petri dishes containing Provasoli enriched seawater (PES) medium (Provasoli 1968). Cultures were maintained at 21 ± 1℃ with an illumination regime of 12 : 12 h light-dark (LD), with a photon flux density of 15 μmol m-2 s-1. Germlings were obtained either from swarmers or from outgrowths of the endophytes from infected thalli. They were subsequently segregated into unialgal cultures and maintained under the above-mentioned conditions with weekly changes of the medium. A 2.5% germanium dioxide solution (Across Organics, Gell, Belgium) dissolved in water was added to avoid diatom contamination (Lewin 1966; Christensen 1982).
Infections of R. pseudopalmata by selected isolates of L. elsbetiae initially established from swarmers were experimentally carried out. Eight to ten 1-1.5 cm long fragments of fronds of the host were placed into plastic Petri dishes. Two replicates of each isolate were incubated under laboratory conditions during a period from 2 to 3 weeks.
Cytomorphometry was carried out using a stereoscop-ic microscope Wild-Herbrugg and an inverted micro-scope Nikon Eclipse TE 300, with anoptral phase contrast and differential interference contras) and with an incor-porated camera Nikon FDX 35. Either the presence or absence of epi-endophyte filaments was determined under light microscopy in semi-thin sections of thalli of R. pseudopalmata. In order to obtain semithin sections, thalli were fixed in 2.5% glutaraldehyde (Fisher Bioreagents, Fair Lawn, Ny, USA) in seawater for 2 hr at 4°C, and postfixed in 1% (OsO4) Osmium tetroxide (Across Organics, Gell, Belgium) in sea water for 2 hr at 4°C. The material was dehydrated in a graded acetone series (Merck, Darmstadt, Germany) and embedded in Spurr’s low viscosity resin (SPi Supplies Divisi, of Strutur pProe, Inc., West Chester, PA, USA). Sections were obtained with glass knives on a Reicher Ultracut OM U2 ultramicrotome (Reicher, Vienna, Austria). The resin was removed using a metallic sodium, benzene and methyl alcohol solution (Hayat 1986). Sections were stained with a combination of colorants, namely haematoxiline (Biopur, Rosario, Argentina) - malachite green (Biopack, Buenos Aires, Argentina) - basic fucsine (Britania, Buenos Aires, Argentina) (1 : 1 : 1) (Berkowitz et al. 1968).
Chromosome counts were made using unialgal cultures of L. elsbetiae. Thalli were fixed either in 1 : 3 mixture glacial acetic acid (Cicarelli Laboratorios, San Lorenzo, Argentina) /absolute ethanol (Cicarelli Laboratorios) or in 6 : 3 : 1 mixture formaldehyde (Cicarelli Laboratorios) - absolute ethanol (Cicarelli Laboratorios) - glacial acetic acid (Cicarelli Laboratorios) at 5℃ during a period of 2 to 24 h. Postfixation was carried out with 70% ethylic alcohol (Cicarelli Laboratorios). The material was subsequently hydrolyzed for 30 min in 1 N hydrochloric acid (HCL) (Cicarelli Laboratorios) at room temperature, stained with Schiff stain in darkness for 2 h (Johansen 1940), bleached during 20 min in a 1 : 3 : 3 mixture of sodium metasulphite (Cicarelli Laboratorios): 1 N HCL: distilled water, washed with distilled water for 30 min, and finally mounted in a drop of a 2% acetic acid solution of ferric haematoxylin (Biopur) with added iron acetate (Biopur) (Nunez 1968).
L. elsbetiae filaments were fixed in 2.5% glutaraldehide (Fisher Bioreagents)-seawater at 5℃ in cacodilate buffer (Biopur) for 2 h. They were subsequently mounted on slides covered with 0.5% poly-D-lysine and dehydrated in a graded acetone series. Samples were finally critical point dried during 1 h, coated with gold, and observed with a Jeol 35 CF scanning electron microscope (Jeol, Tokyo, Japan).
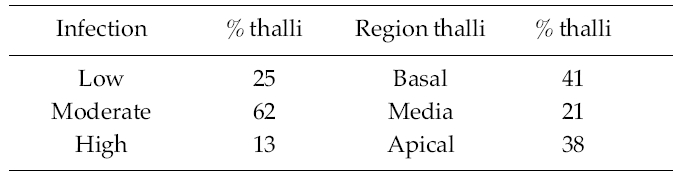
The percentage of infection of R. pseudopalmata thalli by L. elsbetiae was 34%. A 25% of the infected thalli presented a low, non-symptomatic level infection, whereas a 62% and a 13% of them exhibited respectively moderate and high indexes of infection and presented dark patches on the host’s surface as a visible symptom of the presence of dense bushes of L. elsbetiae filaments (Table 1).
A 41% of the infected fronds R. pseudopalmata presented L. elsbetiae individuals in the basal region, whereas a 21% and a 38% of them exhibited them in the median and the apical regions respectively (Table 1).
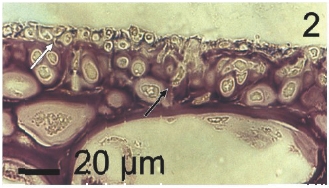
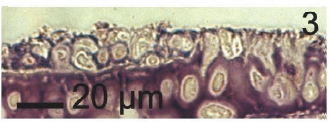
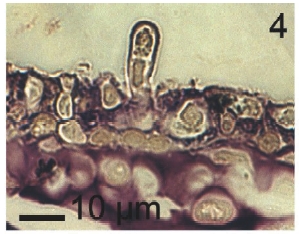
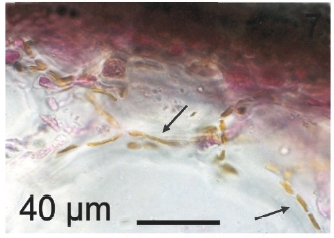
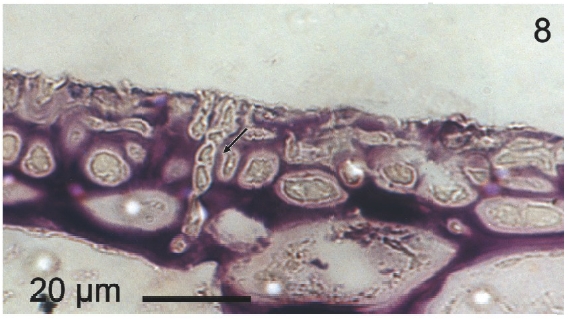
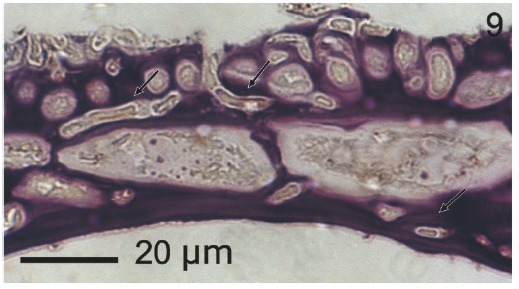
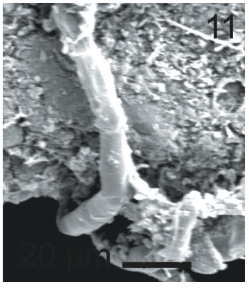
Vegetative filaments of L. elsbetiae that infected R. pseudopalmata thalli were uniseriate. Those growing on the cuticle (Fig. 2) were postrate, regularly arranged with thick cellular walls and in some sectors observed in 2 or 3 levels (Fig. 3). Also erect filaments were observed formed by few cells (Fig. 4). Those developing amid epidermic cells of the host (Figs 5, 6) also formed a fine net flanked by both cortical and medullar cells (Fig. 7). In both areas, cortical and medullar, vegetative filaments did not penetrate into the host’s cells. Filaments were irregularly branched and showed apical growth (Fig. 8). They were formed by cylindrical cells (Fig. 7) 50-60 μm in long and 7-13 μm in diameter. These cells contained 1 or 2 discoid or irregularly elongated chloroplasts with a single pyrenoid. Filaments invaded the host tissues penetrating through intercellular spaces of the cortical region (Fig. 9) up to the medulla (Fig. 8). Reproductive structures of L. elsbetiae on the host were absent. Under scanning electron microscope the cells of L. elsbetiae exhibited delicate surface ornamentation on the walls (Figs 10, 11).
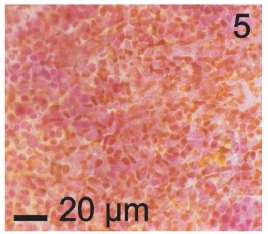
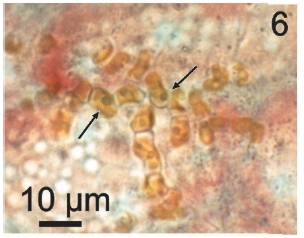
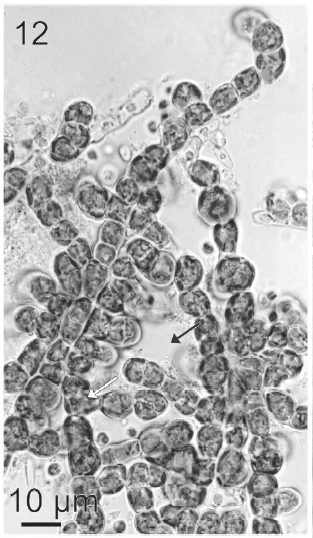
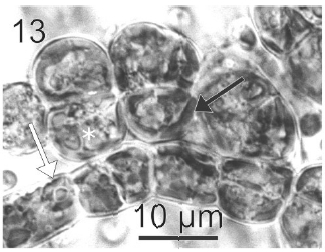
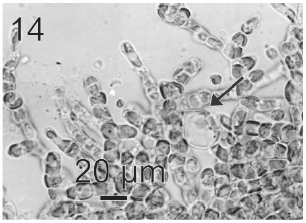
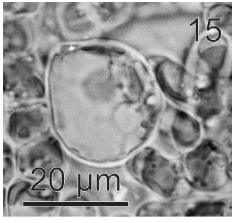
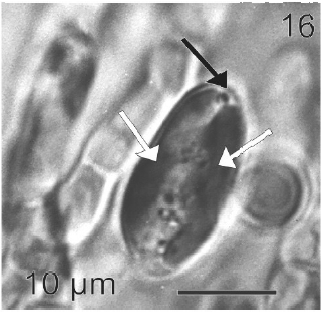
After a week of growth, sporophytes exhibited a postrate basal system and also a less developed erect system (Fig. 12). Both system presented diffuse growth. Cells of the first system were ovoid with a length of 10-20 μm (12.6 ± 2.5 μm, n = 30) and a wide of 7-13 μm (10 ± 1.5 μm, n = 30). These cells presented various parietal, discoid or ribbon-shaped plastids with pyrenoids (Fig. 13). The second system were formed by cylindrical cells, 5.4-21 μm (11 ± 33 μm) (n = 30) in length, 7.7-13 μm (8.7 ± 1.4 μm, n = 30) in wide. Macrozoosporangia originated from vegetative cells of the postrate system were observed (Fig. 14). They were both intercalar and terminal, presented an ovoid shape and a length of 30-50 μm and a wide of 18-20 μm (Fig. 15). In each macrosporangium a single macrozoospore was formed (Fig. 16), which was released through an apical aperture. Macrozoospores presented positive phototaxis. They were pyriform, 18-20 μm in length and 10-13 μm in wide and exhibited two plastids and stigma (Fig. 16). Flagella were of equal lengths and were laterally inserted adjacent to the stigma.
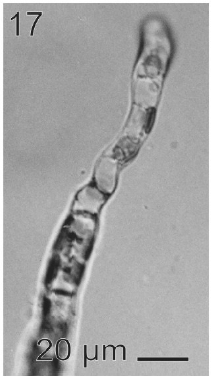
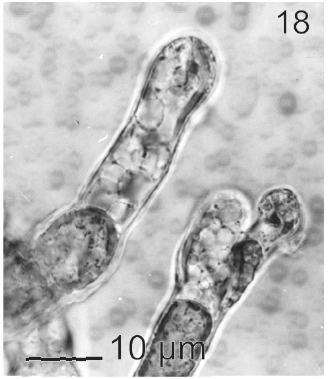
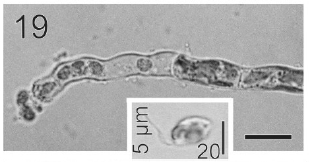
In most of the thalli uniseriate plurilocular zoosporangia were observed (Fig. 17). They were sessile with ovoid to cylindrical shape and they presented ca. 40 μm in length and 10-13 μm in wide. These zoosporangia were formed in terminal vegetative cells. In them unispores or microzoospores were observed (Fig. 18). The unispores were released from loci through an apical pore (Fig. 19) they presented an ovoid chloroplast with a stigma in its anterior end (Fig. 20). They were biflagellate and 4-8 μm in wide. More of 18 microspores per every unilocular sporangium were formed (Fig. 18). These microspores were smaller than macrospores and presented a faster motion. Macrosporangia and uniseriate plurilocular zoosporangia were formed in different thalli.
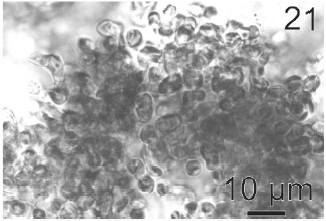
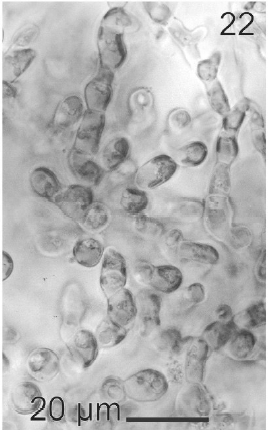
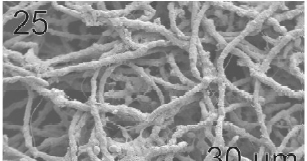
Gametophytes of L. elsbetiae were filaments with diffuse grow, branched with a branch pattern alternate or opposite (Figs 21, 25). Basal cells were 8-26 μm (11.7 ± 4 μm, n = 50) in length and 3.7-11 μm (9.6 ± 3.7 μm, n = 50) μm in wide. Distal cells were 3.7-43.6 μm (15.2 ± 3.5 μm, n = 50) in length and 3.7-25.9 μm (8.9 ± 1.7 μm, n = 50) in wide (Fig. 22). Both type of cells had two or three plastids with pyrenoids (Fig. 22).
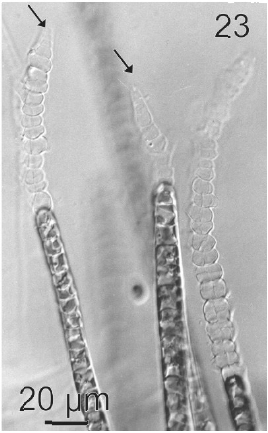
Gametangia were plurilocular, uni or biseriate and lateral. They were 31.4 μm (15-46 μm) in length and 12.9 μm (7.7-25 μm) in wide (Figs 23, 24). Gametangia loci were 5- 6 μm in length and 7-8 μm in wide. When mature they contained 2 to 6 isogametes with a size 4-6 x 4-6 μm. Gametes presented 1 or 2 plastids and stigma. They were released from a pore located in the apical end of gametangia (Fig. 23). Gametes showed positive phototaxis.
Chromosomes were ovoid. L. elsbetiae’s sporophytes presented a number 2n = 10 (Figs 26, 27) and gametophytes n = 5 (Figs 28, 29).
Kawai and Tokuyama (1995) considered L. elsbetiae a representative of the order Ectocarpales because of its morphology and plastid type. Peters and Ellertsdottir’s (1996) observations of sexuality, a slightly heteromorphic life history and isogamy are congruent with this criterion.
This work represents the first report of L. elsbetiae in America, since this species only had been reported for Japan (Kawai and Tokuyama 1995) and Germany (Peters and Ellerrsdottir 1996). In 1995, Kawai and Tokuyama described L. elsbetiae as a new endophyte kelp of northern Japan. In Europe L. elsbetiae was detected infecting Laminaria saccharina (Linnaeus) Lamouroux with high prevalences (Ellertsdottir and Peters 1997). In Muroran, Japan, L. elsbetiae is common in adult Laminaria sporophytes, although they were not found in Costaria Greville and Undaria Suringar, which also grow in the same locality (Kawai and Tokuyama 1995).
Kawai and Tokuyama (1995) observed under culture condition that L. elsbetiae also infected sporophytes of Saccorhiza Bachelot de la Pylaie, which is less related to Laminaria than Costaria and Undaria, and suggested that probably L. elsbetiae did not show high host specificity and was potentially able to infect many other Laminarialean species in nature. The presence of L. elsbetiae on R. pseudopalmata confirmed their predictions of low specificity of this species, not only by its capacity of infection to Laminarialean species, but also to seaweeds of different algal classes. Nevertheless here, in Argentina L. elsbetiae was never observed on Undaria pinnatifida (Harvey) Suringar sporophytes whose fronds, in turn, were infected by another brown endophyte, Laminariocolax aecidioides (Rosenvinge) Peters (Gauna et al. 2009).
L. elsbetiae infection is widespread throughout different populations of R. pseudopalmata from Argentina (Gauna 2005). In this particular Patagonian population, the infection levels observed did not reach the high intensities reported by Peters and Ellertsdottir (1996) in populations L. saccharina, with prevalences there of ca. 93% in the 53% of the population. In fact, here only a 13% of the R. pseudopalmata population presented the highest level of prevalence.
We found here differences with the representatives of L. elsbetiae of Japan (Kawai and Tokuyama 1995) and Germany (Peters and Ellertsdottir 1996). Given the great plasticity of members of Ectocarpales (Muller 1967) we considered them inside a normal intraspecific variability. Vegetative cells of sporophytes of L. elsbetiae isolated from L. saccharina (Peters and Ellertsdottir 1996) were a little shorter than those of the Patagonian isolates and their cellular features are in agreement with those described by Kawai and Tokuyama’s (1995) in Japan. On the contrary, when comparing reproductive structures we found conspicuous differences, i.e., unilocular sporangia of L. elsbetiae growing on R. pseudopalmata were decidedly smaller than those of L. elsbetiae from Helgoland (Peters and Ellertsdottir 1996) and Japan (Kawai and Toyuyama 1995). In addition, in the sporangia from the Argentinian sporophytes not more than 18 microspores were observed, while in sporophytes from L. saccharina (Peters and Ellertsdottir 1996) zoospores are more than 68. And finally, macrosporangia of thalli isolated from R. pseudopalmata are smaller than those observed by Kawai and Tokuyama (1995) and consequently macrozoids of the Patagonian thalli were shorter than those observed in Japan. With relation to the cellular dimensions of gametophytes, they are in agreement with the dimensions of central cells of the thalli of Patagonian L. elsbetiae. The loculi of the gametangia observed in our samples were larger than those observed in L. elsbetiae from Helgoland.
We have not observed the formation of reproductive structures of L. elsbetiae in nature during summer. No fertile structures were also observed during summer and other seasons (winter and autumn) by Peters and Ellertsdottir (1996). These authors observed the formation during May (spring in the North hemisphere) of macrosporangia that protruded slighty from the meristoderm of the host and they hypothesize that during this season L. elsbetiae rapidly spreads in the host population by means of direct reproduction of sporophytes.
Despite that we did not observe fusion of gametes, by means of chromosome counts we could find out in the present populations of L. elsbetiae the presence of two heteromorphyc generations with both haploid and diploid levels of ploidy indicative of a diplobiontic life cycle. Gametophytes were not clearly observed in the field but they were experimentally isolated from hosts’ fronds. The firm adherence of them to the substrate and the fact that they never developed from isolates started with filaments from internal tissues of infected hostsmay indicate that the idea of Peters and Ellertsdottir (1996) in the sense that gametophytes of L. elsbetiae has epilithic or epiphytic but not endophytic growth habit is correct.
Field and experimental observations indicate that the infection of L. elsbetiae is transferred from one frond of R. pseudopalmata to another by spore discharge into the surrounding medium. Collected material, on which recently settled zoospores and germlings were found, the subsequent development of the infection in areas of the fronds took place. As Heesch and Peters (1999) did, we observed that L. elsbetiae successfully enter into the host and developed as interstitial filaments, but the mode of entry into the host tissue has not been accurately verified.
The nature of interactions between R. pseudopalmata and L. elsbetiae remains to be undoubtedly established. In some instances L. elsbetiae caused little perturbation of the R. pseudopalmata tissues. In other cases we have noted the complete replacement of the host cortical tissue. Similar distortions of host have been observed especially by species of Streblonema (Andrews 1976; Yoshida and Akiyama 1979). Epiphytes are usually defined as organisms that grow on plants, but do not derive nutrients from their hosts (Linskens 1976). Linskens (1963) named two kings of epiphytes: holo-epiphytes, organisms attached to the outer layers of the host and amphi-epiphytes, organisms deeply anchored in the tissues of their hosts. Also, Linskens (1963) suggested that the type of anatomical contact is highly variable and that it is determined by the nature of the partners. Endophytism has been defined as the type of symbiosis in which an organism lives within the tissues of a plant host (Correa et al. 1988). Other authors, such as Lewis (1973); Starr (1975); Goff (1982); Lewin (1982); Smith and Douglas (1987) and Douglas and Smith (1989) have also used the term endophytism in the same sense. A wide variety of algae that infect other algae from an anatomical point of view are a continuum between epiphytes and endophytes. Therefore they are named epi-endophytes. In general, epi-endophytes are pigmented algae photosynthetically independent with almost no metabolic relation with their hosts. For that reason many of them can be isolated from their hosts and subsequently be cultivated under laboratory conditions (White and Boney 1969, 1970; Boney 1972; Garbary 1979; Nielsen 1987; Gauna et al. 2009). In this sense the presence L. elsbetiae on R. pseudopalmata could be defined as an epi-endophytic relationship.