Antioxidants are very important not only for the prevention of food oxidation but also for the defense of living cells against oxidative damage (Kim et al. 2003). Antioxidants reduce harmful reactive free radicals and reactive oxygen species (ROS) in cells (Anchana et al. 2005), thereby preventing cancer and heart disease (Yan et al. 1999; Qi et al. 2005). Several synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ) are extensively used because of their excellent efficacy and low cost. However, these artificial chemicals have major side-effect issues including toxicity and DNA damage problems (Choi et al. 1993; Sasaki et al. 2002). Therefore, the identification and isolation of new natural antioxidants from aquatic and terrestrial plants are desirable (Nishida et al. 1996).
Zostera marina L. is an important species in coastal ecosystems because it contributes for nutrient cycling and sediment stabilizer, and provides food stuffs and habitat for many marine organisms such as invertebrates and fishes (Harrison 1882; Moore and Short 2006). Z. marina produces many secondary metabolites (e.g. phenolic compounds) in order to protect itself against microorganisms, epiphytes, and predation (Harrison and Chan 1980; Harrison 1982; Vergeer et al. 1995). As halophytes and mangroves growing in stressful environments make phenolic compounds to suppress the growth of yeast and mold (Bandaranayake 2002), Z. marina produces phenolic contents for protecting itself against Labyrinthula zosterae Porter & Muehlstein, a marine slime mould-like protist (Buchsbaum et al. 1990; Vergeer et al. 1995; Vergeer and Develi 1997). In marine plants, bioactive chemicals such as phenolic compound play important roles for survival and growth, and are now being used in the development of new drugs and health foods for human (Baker 1984; Smit 2004; Katalinic et al. 2006).
Harrison and Chan (1980) reported that methanol extracts of Zostera marina inhibit the growth of various invading microalgae (diatoms and flagellates) and a bacterium (Staphylococcus aureus). Antioxidant activity for the polysaccharide zosterine isolated from Z. marina has been examined by the activation of free radical peroxide oxidation in mice (Khasina et al. 2003) and the activity was compared with two antioxidant drugs (Kolenchenko et al. 2005). Stirk et al. (2007) found that the amount of bioactive chemicals in seaweeds is changed seasonally and regionally due to fluctuation of environmental conditions. For Z. marina methanol extracts, antioxidant activities using DPPH and reducing activity methods, and antimicrobial activities against two human skin pathogens (Staphylococcus epidermidis and Candida albicans) have not been tested yet. It is also worth to note the antioxidant and antimicrobial activities of Korean Z. marina for the first time. Thus, the aim of present study was to examine the antioxidant and antimicrobial activities for Korean Z. marina methanol and fractioned extracts to determine which solvent fraction could be a potential source for developing natural antioxidant and antibiotics.
Preparation of Z. marina extract: Zostera marina was collected at Ihoijin Jangheung, Korea (34° 27’ N, 126° 56’ E), transported within an ice-box to the laboratory, and washed carefully in freshwater to remove sediment and epiphytes. Prior to experiment, samples were freeze dried and stored in a refrigerator below ?15°C. Extractions were made with methanol (MeOH) at room temperature for 24 hrs, filtered (Whatman No. 2), and concentrated by rotary evaporation (RE-111, Switzerland), 35°C below.
The MeOH crude extract (83.40 g) of Z. marina were partitioned into n-hexane, chloroform, ethyl acetate, nbutanol, and water fractions. The partition procedures were as follows; First, MeOH crude extract was dissolved in 200 ml distilled water followed by the addition of 100 ml n-hexane, chloroform, ethyl acetate, and nbutanol, three times respectively (Fig. 1). Subsequently, the dried solvent fractions were dissolved in methanol and stored at below ?15°C.
Chemicals: Chemicals used were Folin-Ciocalteu phenol reagent, DPPH (α-diphenyl-β-picrylhydrazyl), Gallic acid, EDTANa2, trichloroacetic acid, FeCl3.6H2O, K3Fe(CN)6, Na2CO3, FeCl2, n-hexane, chloroform, ethyl acetate, methanol, n-butanol, butylated hydroxyanisole (BHA), and ferrozine [3(2pyridyl) 5,6bis (phenyl sulfonic acid) 1,2,4-triazine].
Total phenolic content: Total phenolic content of Z. marina crude and portioned extracts was quantified according to the methods of Yuan et al. (2005) and expressed as GAE (gallic acid equivalents). Each solvent extract (0.1 ml) of Z. marina was mixed with 2.0 ml of 2% Na2CO3 (dissolved in distilled water) in a test tube and kept at room temperature for 2 min. Subsequently, 0.1 ml of Folin-Ciocalteu’s phenol reagent (50%) was added to the above solution which was kept at room temperature for a further 30 min before absorbance was read at 720 nm.
DPPH radical-scavenging activity: To test radicalscavenging activity, reactions with the DPPH radical were carried out according to the method of Wangensteen et al. (2004). Each solvent extract of Z. marina was made in various concentration (0.1, 0.5, 1, 5, 10, and 20 mg ml?1) and mixed with 2.90 ml of a DPPH solution, which was prepared at a concentration of 0.2 mM in MeOH. After standing for 30 min, absorbance was measured by a UV-VIS spectrophotometer at 517 nm (Biochrom, Libsra S22, England), using MeOH as the blank. The DPPH solution was prepared daily, stored in a flask at room temperature, covered with aluminum foil, and all determinations were performed three times. Antioxidant capacity of the test extracts was expressed as the concentration necessary for reduction of DPPH. Percent radical scavenging was calculated as 100× (Astart ? Aend)/Astart, where Astart is the absorbance before addition of test extract and Aend is the absorbance value after 30 min of reaction time.
Reducing power: Reducing activity of various solvent fractions in Z. marina was determined according to the methods of Yen and Chen (1995). Extracts in phosphate buffer (2.5 ml, 0.2 mol, pH 6.6) were added to potassium ferricyanide (2.5 ml, 10 g L?1) and the mixture was incubated at 50°C for 20 min. Trichloroacetic acid (2.5 ml, 100 g L?1) was added to the mixture and then centrifuged for 10 min at 3,000 rpm. Finally, 2.5 ml of the upper layer was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% aqueous FeCl3, after which absorbance was recorded at 700 nm. Higher optical density of the reaction mixture indicated greater reducing power.
The various solvent fractions of Z. marina were used for assessing antimicrobial activity. Antimicrobial activity was examined against three human skin pathogens; two bacteria (Staphylococcus aureus KCTC 1927, S. epidermidis KCTC 1917) and a yeast (Candida albicans KCTC 7596), using the MIC (minimum inhibitory concentration) method.
The two bacteria were cultured in nutrient broth (8 g L?1, Difco, USA) on agar (15 g L?1), while C. albicans was cultured on Sabouraud dextrose broth (30 g L?1, Difco, USA) on agar (15 g L?1). Suspensions of Staphylococcus aureus, S. epidermidis, and C. albicans were adjusted to an optical density (O.D.) of 0.1 at 600 nm. The cell density of test microorganisms containing 0.1 ml of broth solution was 108 CFU (colony forming unit).
The solvent fractions of Z. marina were dissolved in 10% dimethylsulfoxide (DMSO). Blank DMSO were also placed in separate wells and served as controls. Two-fold serial dilutions of sample solutions were made and final concentrations ranged from 0.125 to 8 mg ml?1. Antimicrobial activity of each solvent fraction against the three skin pathogens was examined by estimating MIC after culture for 24 hrs at 37°C.
Statistical analyses were carried out using STATISTICA version 5.0 software. A one-way ANOVA was used to determine the differences in total phenolic content between the solvent fractions of Zostera marina extract. When significant differences between means were detected, Tukey HSD test was applied (Sokal and Rohlf 1995). Homogeneity of the variance was tested using Cochran’s test (Underwood 1997).
Total phenolic content: MeOH crude extract of Zostera marina was fractionated with various solvents, from which the following were obtained; 4.97 g in n-hexane, 0.34 g in chloroform, 0.35 g in ethyl acetate, 1.08 g in nbutanol, and 50.42 g (60.45%) in water fraction.
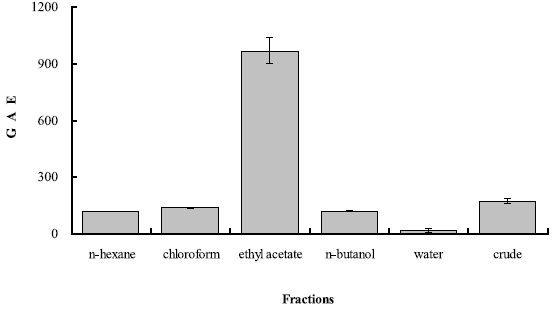
Total phenolic contents were significantly different between solvent fractions of Z. marina (F5, 12 = 244.75, p < 0.001). Total phenolic content was 968.50 μg per 1 mg of ethyl acetate fraction (Fig. 1) which was significantly greater than the other fractions (Tukey HSD test). Also, phenolic content was significantly lesser at water fraction (50.25 μg) than at crude fraction (204.63 μg) of Z. marina, but no differences in total phenolic contents were found between n-hexane, chloroform, n-butanol, and crude solvent fractions (Tukey HSD test). This indicates that the phenolic compounds of Z. marina are mainly contained in non polar extracts rather than in polar solvent extracts.
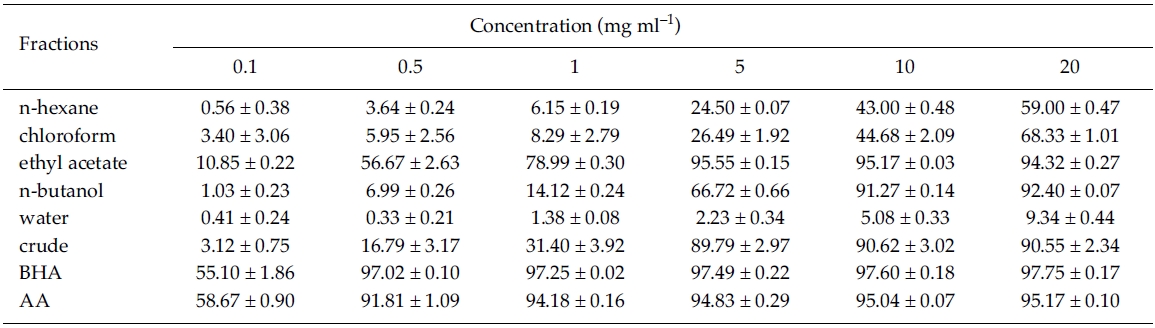
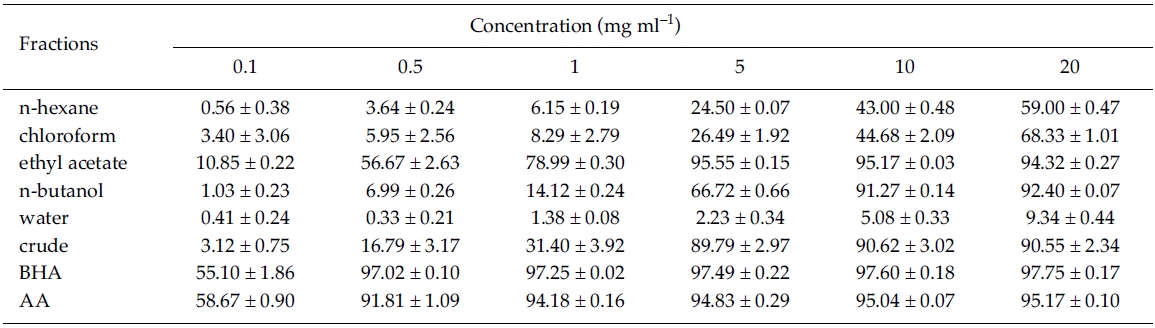
DPPH radical scavenging activity: DPPH radical scavenging activity was enhanced with increasing concentration (between 0.1 and 20 mg ml?1) for the five solvent fractions and crude extracts of Z. marina (Table 1). At water fraction, however, DPPH inhibition activities were very low with less than 10% in the tested concentrations. DPPH radical scavenging activity was significantly greater at ethyl acetate fraction with IC50 values of 0.46 mg ml?1 than the other solvent fractions including MeOH crude extracts in the range of between 0.1 and 5 mg ml?1. At 5 mg ml?1 of ethyl acetate fraction, DPPH scavenging activity was significantly different between treatments (F7, 16 = 905.99, p < 0.001), but it was not significantly different compared to crude extract, BHA, and ascorbic acid (Tukey HSD test; Table 1). As shown in Table 1, the ethyl acetate fraction was most effective in DPPH radical scavenging activity with 95.17%, followed by n-butanol fraction (91.27%) and the MeOH crude extract (90.62%) at a concentration of 10 mg ml?1. In the present study, DPPH radical scavenging activity was relatively high in the non-polar solvent fractions of ethyl acetate and n-butanol.
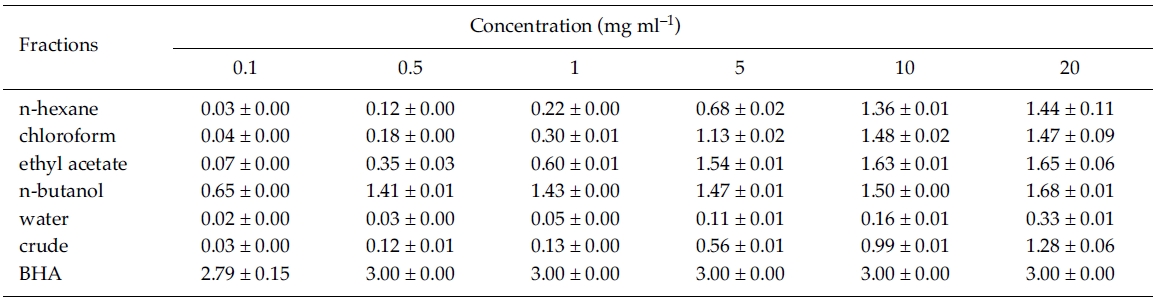
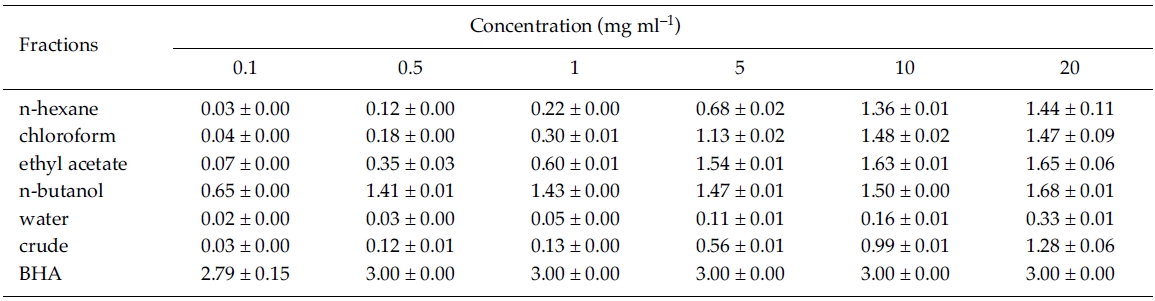
Reducing power: For determination of reducing power, the reduction of Fe3+ to Fe2+ was measured in various solvent fractions of Zostera marina. At 0.1 mg ml?1, reducing power was significantly different between solvent fractions, crude extract, and BHA (F6, 14 = 943.96, p < 0.001), and it was significantly greater at n-butanol fraction than the other solvent fractions (Tukey HSD test). However, reducing power was significantly lower at n-butanol compared to the control, BHA at 0.1 mg ml?1 concentration. At lower concentrations (between 0.1 and 1 mg ml?1), reducing power was stronger in the nbutanol fraction than in the ethyl acetate fraction. However, it was very similar between ethyl acetate and n-butanol fractions at higher concentrations of between 5 and 20 mg ml?1 (Table 2). Thus, the reducing power of the ethyl acetate and n-butanol fractions exhibited higher activity than other fractions.
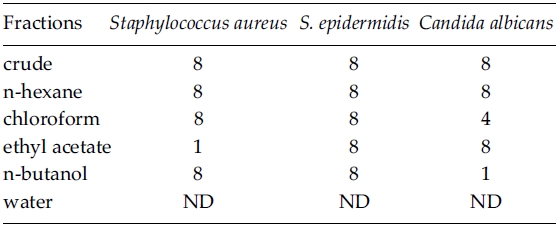
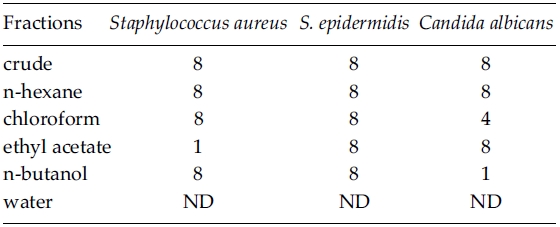
As the MeOH crude extract (10 g dry wt.) of Zostera marina plants was partitioned, the yields of each solvent fraction varied from 0.03 g (0.32%) in ethyl acetate to 1.19 g (11.87%) in water. Antimicrobial activity of Z. marina fractions was in the range of between 1 and 8 mg ml?1 concentration against three human skin pathogens but it was not detected at water fraction (Table 3). Minimum inhibitory concentration for Staphylococcus aureus was found at 1 mg ml?1 in the ethyl acetate fraction. MICs of S. epidermidis were the same at 8 mg ml?1, irrespective of Z. marina solvent fractions. Candida albicans showed different MICs from 1 mg ml?1 in the n-butanol fraction, 4 mg ml?1 in chloroform fraction, to 8 mg ml?1 in the three solvent fractions (Table 3).
Plant products such as flavonoids, cumarins, phenolic acids and terpenoids are reported to have DPPH scavenging activity (Puertas-Mejia et al. 2002). At the same concentration (3×10?5 mol L?1), DPPH quenching activity was stronger in the extract of non-polar solvents (chloroform, ethyl acetate and acetone) than at water, ethanol, or methanol extract in several Rhodomelaceae seaweeds (Yan et al. 1998; Yuan et al. 2005). Jimenez-Escrg et al. (2001) found that DPPH radical scavenging activities are positively correlated with the total polyphenol content in aqueous and organic solvent extracts of brown and red algae. In the present study, phenolic content was approximately 968.50 μg at 1mg of non-polar ethyl acetate fraction of Zostera marina, and DPPH radical scavenging activity (95.55% at 5 mg ml?1) was the highest among various solvent fractions. These results indicate that the presence of compounds with phenolic functional groups in the ethyl acetate fraction.
In the present study, 1 mg ml?1 of n-butanol fraction of Zostera marina appeared to possess 5 times higher reducing activity than at ethyl acetate fraction of Capsicum annuum L. and 35 times greater than at MeOH extract of Palmaria palmata (L.) Kuntze (Kim et al. 2003; Yuan et al. 2005). It is well known that ROS induce oxidative damage to biomolecules like nucleic acids, lipids, proteins, and carbohydrates, and this damage causes cancer, and other disease (Duan et al. 2006). Present results show that ethyl acetate and n-butanol fractions of Z. marina are potential candidates in the development of medicinal compounds relating to prevention of human diseases.
Antimicrobial activity depends on solvent fractions and test organisms. The MICs was the same with 1 mg ml?1 in the ethyl acetate fraction of Z. marina for Staphylococcus aureus and in the n-butanol fraction against C. albicans. For S. aureus, MICs of ethyl acetate fractions were 1 mg ml?1 for Z. marina and 8 mg ml?1 for Neorhodomela aculeata (Perestenko) Musuda (Lee et al. 2006). Harrison and Chan (1980) reported that growth inhibition of several micro-algae and a bacterium, Staphylococcus aureus in Z. marina MeOH extracts. For S. aureus, however, they examined antimicrobial activity with paper disc method and it is impossible to compare the degree of bioactivity between previous and present results. Lee et al. (2006) found that MICs of S. epidermidis were higher (between 16 and 32 mg ml?1) than S. aureus (8-16 mg ml?1) and C. albicans (16 mg ml?1) at four organic solvents (n-hexane, chloroform, ethyl acetate, and methanol) of Neorhodomela aculeata. In the present study, MICs were 8 mg ml?1 for S. epidermidis and 1-8 mg ml?1 for S. aureus and C. albicans in all tested solvent fractions. These results indicate that S. epidermidis is less sensitive than two human skin pathogens in both Z. marina and N. aculeata, and various solvent extracts of Z. marina show higher antimicrobial activity than those of N. aculeata against Staphylococcus epidermidis and C. albicans. Vergeer et al. (1995) reported that the production of phenolic compounds in Z. marina increased when infected by Labyrinthula zosterae, indicating that the production of phenolic compounds is an antimicrobial response.
Research studies have already described the positive relationship among total phenolic content, antioxidant activity, and antimicrobial activity (Velioglu et al. 1998; Da Silva et al. 2006). In the present study, antioxidant (DPPH radical scavenging activity and reducing power) and antimicrobial activities against three human skin pathogens were excellent in ethyl acetate fraction of Z. marina compared to other seaweeds such as Palmaria palmata and N. aculeata. Although the chemical compounds of ethyl acetate fractions from Z. marina have not yet been fully identified, they might be related to phenolic contents which were maximal in ethyl acetate fraction. In conclusion, the ethyl acetate and n-butanol fractions of Z. marina might be useful for developing natural antioxidants for many human diseases and new antibiotics against human skin pathogens but more researches on the isolation and identification of the bioactive compounds are required.