Antarctica and the surrounding waters which form a part of Southern Ocean has a significant influence on the global climate. Southern Ocean is characterized by latitudinal fronts marked by steep changes in sea surface temperature, salinity as also biological productivity (Orsi
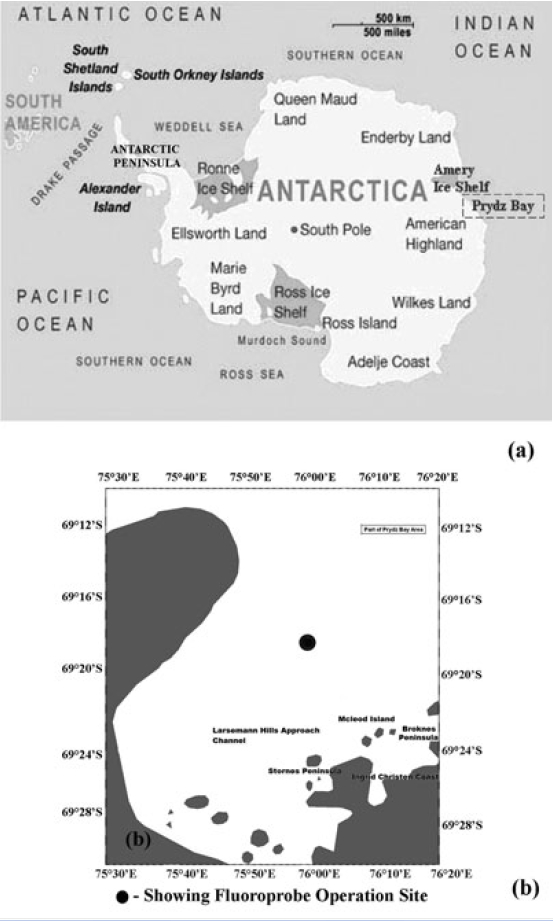
Prydz Bay, covering an area of about 80,000 km2, is a triangular-shaped embayment in the Indian sector of Southern Ocean (Stagg 1985) (Fig. 1). This is situated between West Ice Shelf (around 80°E) and Cape Darnley (70°E) and the region off the Mawson coast which is often ice-covered similar to the two major Antarctic coastal seas - the Weddell and Ross Sea. In summer season, Antarctic Surface Water mass in Prydz Bay is subdivided into summer surface water and continental shelf water. Summer surface water is relatively warm water having temperature 0-2°C and salinity 33.5-34 psu which is attributed to summer heating and melting (Smith
Kang and Fryxell (1991) opined that surface layer depends upon local sea-surface conditions and thermohaline structures which is regulated by horizontal advection through wind-forcing. The base of the summer surface layer has been assumed to correspond with the top of the seasonal thermocline in the 20-50 m depth range. The thickness of the surface layer generally increases to the north outside Prydz Bay, consistent with its relationship to the density of the ice coverage (Smith
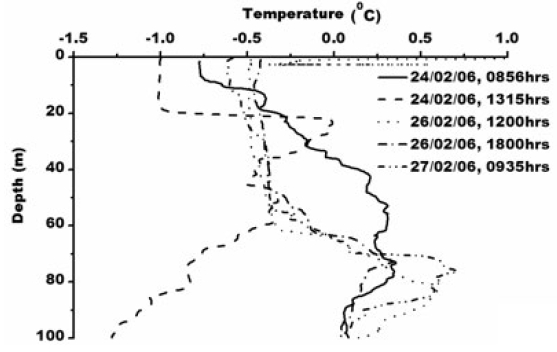
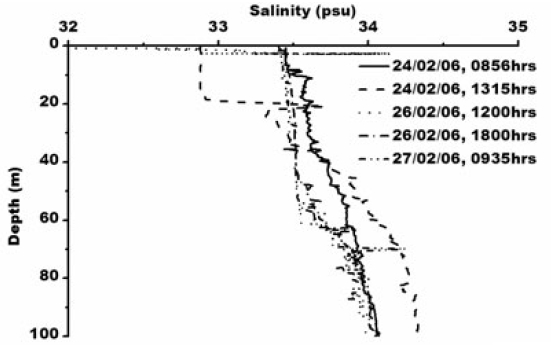
Smith et al. (1984) observed icebergs off Princess Elizabeth Land moving southwest, while those calved from the Amery Ice Shelf normally follow the western periphery of Prydz Bay toward Cape Darnley. Observation of pack ice movement, iceberg tracks and buoy trajectories near the Antarctic coast provide additional evidence of a westward coastal current (Swithinbank et al. 1977; Tchernia and Jeannin 1980). Physical parameters measured in the Prydz Bay indicate a typical thermohaline structure as also mixing in that area by periodic upwelling processes associated with tides and continental shelf waves (Middleton and Humphries 1989). Nunes Vaz and Lennon (1996) reported based on the hydrographic data collected during the 1980’s that this area is a production site for dense water. During the present study XBT operations (type: T-7; accuracy: ±0.15°C; depth resolution: 0.65 m) were carried out upto 100 m. The sea surface temperature was recorded using a bucket thermometer (accuracy: ±0.1°C) whereas Portable CTD (make: SBE 9/11 plus, Sea-Bird Electronics, USA; temperature accuracy: ±0.001°C, conductivity: ±0.0001 sm-1 and depth ±0.005%) was operated for deeper depth. Temperature varied from ~ -1.0 to 1°C in the water column/surface (Fig. 2) and salinity varied from 32.88 to 34.32 psu (Fig. 3).
During the Special Expedition to Larsemann Hills and Southern Ocean (2006) in the summer to winter transition (last week of February 2006) R/V Akademik Boris Petrov was off the Prydz Bay area for undertaking multibeam swath bathymetry of the channel to the station site. A submersible fluorescence probe (FluoroProbe, bbe- Moldaenke, Kiel, Germany) was operated up to 100 m depth for in situ measurement of total chlorophyll and phytoplankton concentration. A total of seven stations at different time intervals between 24-27 February, 2006 were carried out at 69°18’S, 76°00’E. The FluoroProbe for algal differentiation uses 5 light emitted diodes (LED) for fluorescence excitation. The LEDs emit pulsed light at fixed wavelengths (450 nm, 525 nm, 570 nm, 590 nm and 610nm). Fluorometric emission is measured at 680 nm by photomultiplier at an angle of 90° to the exciting light source (Buetler et al. 2002). The probe is connected online mode to a laptop from which it is operated and to which the data on depth and phytoplankton composition are sent every second. The division of Chlorophyceae (Green algae) shows a broad maximum of fluorescence at the 450 nm LED that corresponds to chlorophyll-a and chlorophyll-b excitaion. The Cyanophyceae (Blue-green algae) pigments are characterized by maximum excitation at 610 nm caused by photosynthetic antenna pigment phycocyanin. 525 nm region for Bacillariophyceae (Diatoms) originates from xanthophylls, fucoxanthin andperidin for the division of Dinophyceae. The maxima at450 nm is caused by chlorophyll-a and chlorophyll-c.Group Cryptophyceae originates from phycoerythrin at570 nm. For precision of the Fluoroprobe measurementsand calibration see Buetler et al. (2002). We have reported 15N based production measurements in the SouthernIndian Ocean elsewhere (Personal communication). Wemeasured the nitrate, ammonium and urea uptake ratesand f-ratio for the surface waters of Prydz Bay. 250 ml ofsamples were separately collected for nutrient measurements(for details of experimental techniques reference ismade to Kumar et al. (2004).
Total chlorophyll concentration ranges from 0.03-14.87 μg L-1 (Fig. 4). Maximum chlorophyll concentration of 14.87 μg L?1 was observed at 1303 h, 24th February. The chlorophyll maxima is recorded at 15 meter water depth. One of the key characteristics of the chlorophyll ‘a’ is its fluorescence. Fluorescence of photosynthetic pigments is partly dependent on the physiological status of the cell and its photosystems, which is affected by factors, such as life history of the cell, nutrients availability and light conditions (Kiefer 1973; Vincent et al. 1984; Soohoo et al. 1986). Photosystem II (PSII) is mainly responsible for chlorophyll fluorescence which consists of a peripheral and core antenna. The former contains a species-dependent light absorbing pigment, the later an evolutionary conserved molecule of chlorophyll (Buetler et al. 2002). Fluorescence emission of PSII measured around 685 nm is generally accepted as a measure of chlorophyll content of algae in aquatic system (Yentsch and Menzel 1963; Holm-Hansen 1965). Higher chlorophyll concentration in oceanic water of Prydz Bay is attributed to higher primary productivity and eutrophic nature of Antarctic waters. Persistence of abundant chlorophyll-a in the coastal waters in the eastern part of Prydz Bay could be attributed to cooler coastal waters which are generally more productive (Nicol et al. 2000) which substantiate our study as maximum chlorophyll concentration of 14.87 μg L-1 occurs where water temperature is -1°C at surface to 20m depth indicating upward movement of water due to local wind forcing. This subsurface cold water is due to the winter water which occurs as residue from the previous winter mixed layer, limited by seasonal warming and freshening (Park et al. 1998). Chlorophyll less than 14.87 μg L-1 occurs where temperature is greater than -1°C and shows negative correlation with chlorophyll (R2 = -.29, -.79, -.86, -.69 for temperature station 1, 3, 4 and 5 respectively, Fig. 2). Chlorophyll-a concentration in the euphotic zone increases and the critical depth decreases due to increased attenuation of PAR, as light levels may become limiting for phytoplankton growth (Strutton et al. 2000). Earlier study also suggests that the chlorophylla concentration in the Prydz Bay is high (Gibson and Trull 1999). They reported chlorophyll concentration >15 mg m?3 during the mid summer (January).
Mongoni et al. (2004) have reported 2.5 fold higher chlorophyll-a concentration of 102 and 206 mg m?2 during January and February respectively in Ross Sea. They observed broken pack and melting ice which was coloured by an extensive algal biomass suggesting that the phytoplankton was a result of seeding from ice algal communities. Higher level of chlorophyll concentration may be attributed to coverage of summer ice in late summer season when freezing starts. In Indian Sector of Southern Ocean, 63°S onwards to Antarctica, melting/freezing processes are more prominent in late austral summer (Srivastava et al. 2007). During freezing, salinity increases as ice discards salt; conversely melting decreases the salinity due to input of fresh water (Archambeau et al. 1998). The variable concentration of chlorophyll at the same location in different timings could be attributed to melting/ freezing processes. During melting, a stable surface layer forms with ample amount of nutrients and warmer water temperature favouring the growth of phytoplankton (Mitchell and Holm-Hansen 1991). This is further substantiated by higher Total N uptake rate (~12.3 mmol N m?2 d?1). The photic zone concentrations of nitrate, ammonia and urea are 7.70, 3.30, and 1.27 mmol N m?2 d?1 respectively. In terms of carbon, the total carbon uptake during late austral summer in the Antarctic coastal zone is 981.5 mmol N m?2 d?1. The f-ratio here is 0.63. High carbon uptake rate in coastal regions of Antarctica was also reported by other authors (Treguer and Jacques 1992). The production in the region is high as the coastal region receives ample nutrients from the Antarctic continent. These nutrients are derived through the coastal continental erosion and contain significant amount of iron along with other major nutrients.
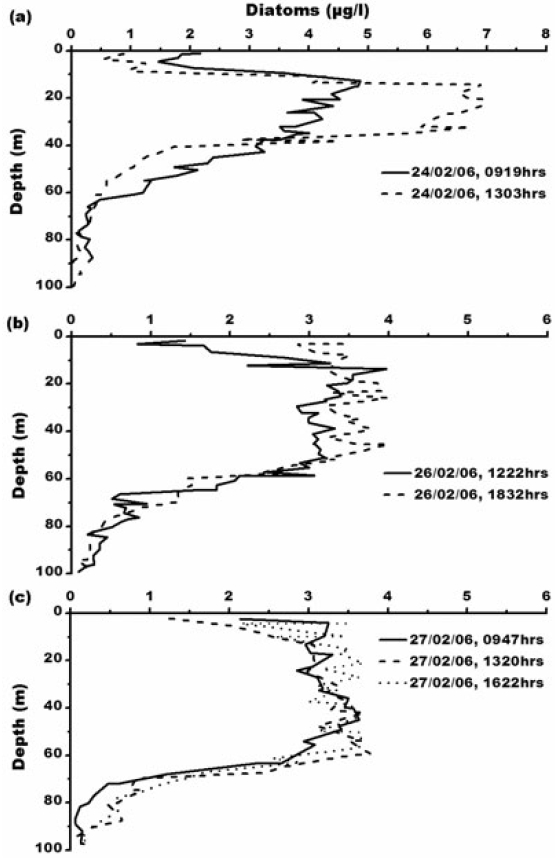
Diatom concentration ranges from 0.01-6.94 μg L?1. Maximum diatom concentration of 6.94 μg L?1 has been observed at 1303 h, 24th February, 2006 (Fig. 5). Elevated levels of diatom concentration is due to high nutrient concentration. Earlier studies also reported diatom dominance in Prydz Bay (Kang and Fryxell 1991; Kopczynska
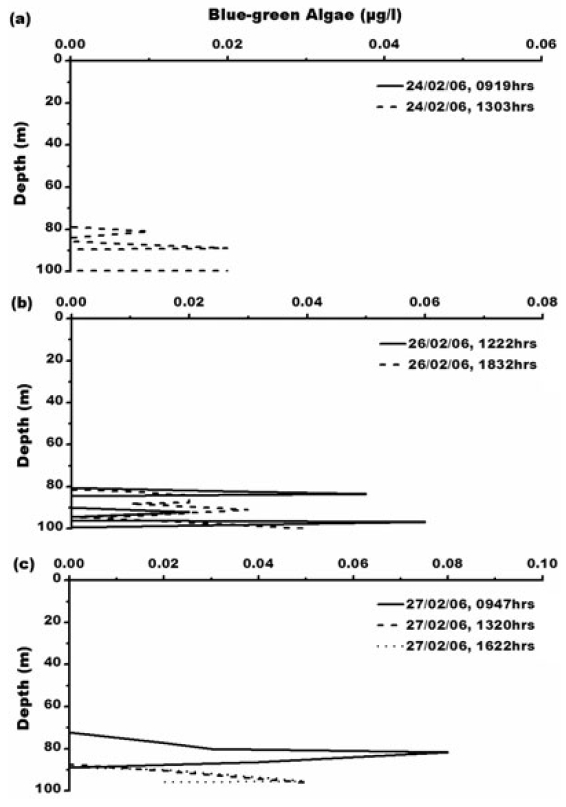
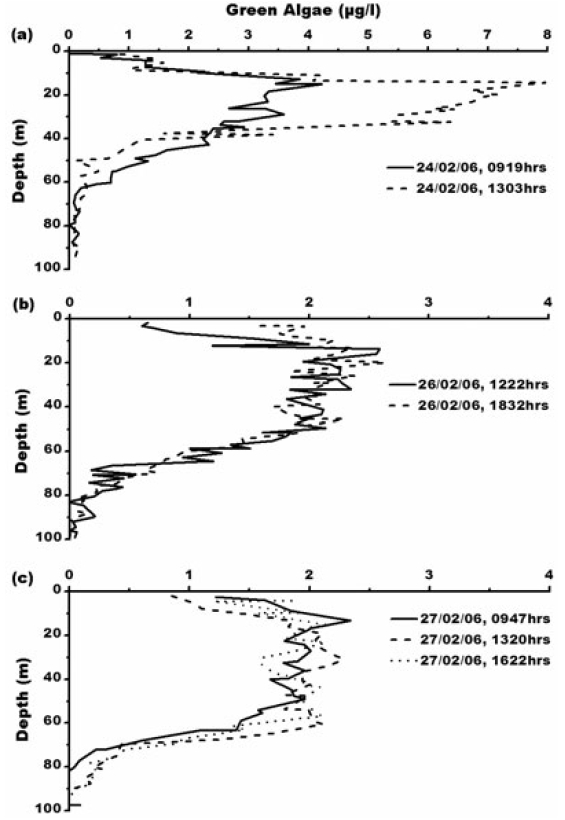
Concentration of blue-green algae (BGA) varies from 0.01-0.08 μg L?1. Its maximum concentration of 0.08 μg L?1 is at 0947 h, 27th February, 2006 (Fig. 6) which is between 65-85 m depth where water is comparatively warmer with temperature of 0.6°C with maximum salinity- 33.3 psu. Green algae concentration ranges from 0.01- 7.97 μg L?1. The maximum concentration of green algae (7.97 μg L?1) is at 1303 h, 24th February, 2006 (Fig. 7). Green algal concentration is following same trend like chlorophyll concentration suggesting that lower temperature of -1°C is favouring their growth (Fig. 7a). Concentration of green algae is high compared to that of blue-green algae which is due low sea surface temperature and also widespread distribution of chlorophyll-b (Bidigare
The Prydz Bay studied area is characterized by abundant phytoplankton assemblage dominated by diatoms. Maximum chlorophyll concentration occurs where water temperature is lowest (-1℃) from surface to 20 m depth due to upward movement of winter water. An adequate light availability associated with vertical stability seems to favour phytoplankton blooms in Antarctic waters. In coastal upwelling regions, the dominant primary producers are diatoms which contribute to high siliceous ooze as they have high sinking velocities and sink either as individuals, aggregates, mats or in fecal pellets. The particular area has high productivity (981.5 mgC m?2 d?1) and high