Pesticides are chemicals used for crop protection and pest control and are speculated to be the most widely distributed contaminants in the environment over the last century. Although it is difficult to obtain precise quantities concerning their production and usage, millions of tons of pesticides are produced and spread annually all over the world.1) Given this widespread use, they are detected using various environmental matrices such as water,2) soil,3) and air.4)
Pesticides are generally classified according to the type of pest they are intended to control or kill, with many varieties that include insecticides, miticides, fungicides, herbicides, algicides, rodenticides, avicides, larvicides, and germicides.5) Pesticides can be composed of inorganic substances, organometallic compounds, volatile organic compounds (VOCs), semi-VOCs, and non-VOCs, whereas the most conventional pesticides are semi-VOCs or non-VOCs.4) Pesticides are divided into many classes, of which the most important are organochlorine (OC) and organophosphorus (OP). Due to the long residence time of these substances in the environment, many pesticides used in the fields end up in water resources. Surface water contamination may have ecotoxicological effects upon aquatic organisms, as well as human health, if used for public consumption.6,7)
In order to minimize exposure, development of effective treatment technologies for direct remediation at the contaminated sites is paramount. Conventional techniques that include physical and/or chemical treatments8) are typically used for pesticide removal. However, these are often expensive and produce hazardous by-products that must be shipped to landfills for disposal. In the past decade, the use of phyto-processes to remediate contaminated water has gained popularity is a new green evolution technology that is cost-effective and eco-friendly, as well as an efficient in situ technology for a variety of pollutants, including nutrients and heavy metals.9-11) Indeed, some plants possess a natural ability to absorb and hyperaccumulate trace elements in their tissues.12,13) Phytoremediation is also being studied as a sound approach to degrade persistent organic pollutants (POPs), including pesticides.14-17) Nevertheless, little information is available on the behavior of pesticides in aqueous solutions that involve the plant-uptake mechanism; information on compound plant/water partition coefficients and kinetic uptake parameters are only known for a very few species.
The objective of this work was to study the behavior of OP and OC pesticides in aquatic systems, including the remediation efficiency with plant and plant-pesticide uptake, as well as the behavior of pesticide translocation in the plant organs. The four OPs (diazinon, fenitrothion, malathion, parathion) and two OCs (dieldrin and hexachlorobenzene) were chosen as models because of their common use as active ingredients in pesticide products and their distinctive chemical structures that represent the variety of OP and OC pesticides. Although it is apparent that the initial behavior of a chemical in the environment is often affected by formulation, the effects of pesticide species on predicting its behavior in phytoremediation techniques have not been widely studied. The term “migration factor” of pesticides (
2.1. Aquatic Plant and Nutrients
Cultivated plants (
[Table 1.] Characteristics of nutrient solution
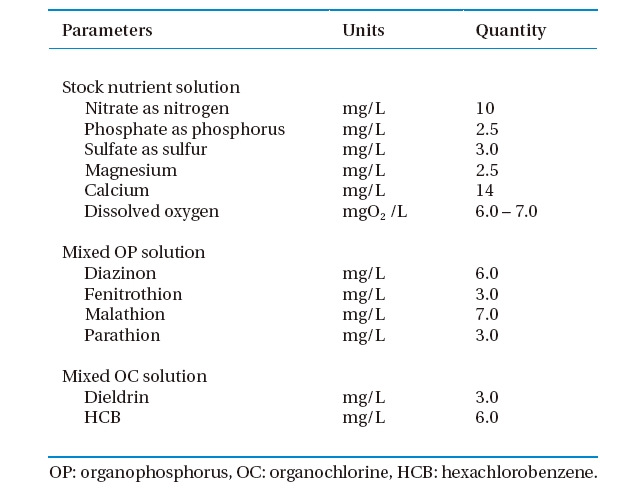
Characteristics of nutrient solution
2.2. Stock Pesticide Solutions
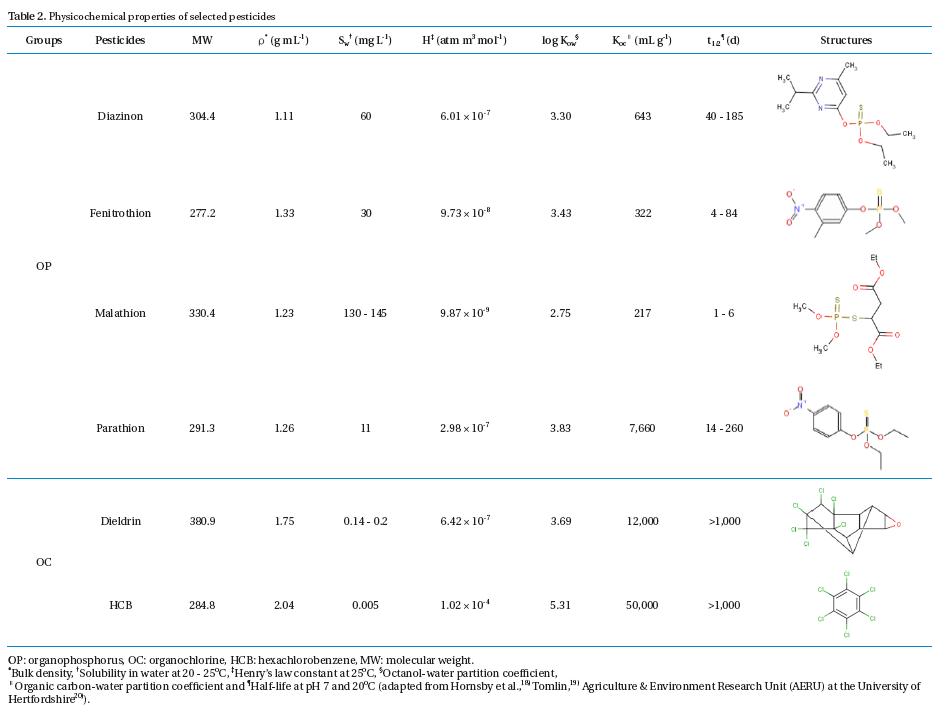
Analytical standard diazinon, fenitrothion, malathion, parathion, dieldrin, and HCB (all assays >;99.9% purity) were purchased from Sigma-Aldrich (Yongin, Korea). Individual pesticide stock solutions were prepared in a methanol solution (Sigma- Aldrich, assay >;99.8%) and stored at -12℃ until use as preparing pesticide medium solutions and standard pesticide solutions for gas chromatography/mass spectroscopy (GC/MS) calibration and interpretation. The physicochemical properties of the selected OP and OC pesticides are given in Table 2.
2.3. Pesticide Solutions for PRs
The OP solution (Table 1) consisted of a mix of diazinon, fenitrothion, malathion, and parathion and was prepared by diluting the appropriate volumes from the individual stock OP pesticide solutions with a nutrient solution (Section 2.1). The method was similar to a preparation of an OC solution consisting of mixed dieldrin and HCB.
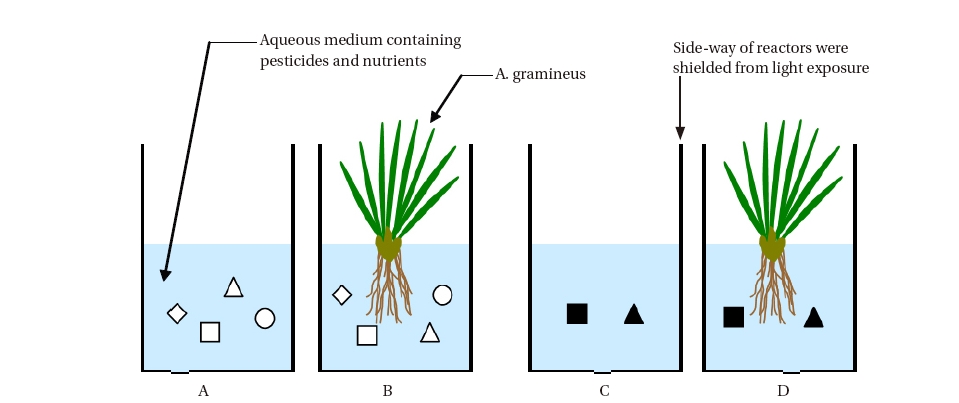
The PRs were made of glass materials with dimensions of ?105 mm × 150 mm (diameter × height), accommodating a volume of 0.5 - 1.0 L. Fig. 1 shows the design and setup of the PRs in the laboratory.
2.5. Sample Extractions and Analysis
2.5.1. Extraction of Pesticides in Aqueous Solutions
The 5.0 mL aqueous samples inside the PRs were sampled daily and immediately extracted with 5.0 mL of n-hexane. The extracted solutions were dehydrated with sodium sulfate, filtered by syringe filters (Whatman glass microfiber filter, grade GF/C 0.45 μm), and analyzed by gas chromatography and mass spectroscopy (GC/MS- QP2010 Plus; Shimadzu Corp., Kyoto, Japan).
2.5.2. Extraction of Pesticides in Plants
In order to determine pesticide distribution in the plant tissues, one-third of the plants were sampled at the end of the first batch tests, while the remaining plants in the PRs were collected at the end of the second batch tests. The plants were separated into three parts (leaves, rhizomes, roots) and recorded for wet weight biomass. Subsequently, they were dried at 35℃ for 72 h in an air recirculation oven (OF-22; Jeio Tech, Kimpo, Korea). The dried plant organs were recorded for their weights and ground into a fine powder. The pesticides in the powder samples were extracted by liquid-liquid (L-L) extraction using ethyl acetate (EA) as the solvent extractor. The extracted solutions were then filtered using Whatman GF/C filters and analyzed by GC/MS.
2.5.3. Extraction of Pesticides in Suspended Matter
At the end of first and second batch tests, the suspended matter inside the PRs were collected by filtering all aqueous solutions
[Table 2.] Physicochemical properties of selected pesticides
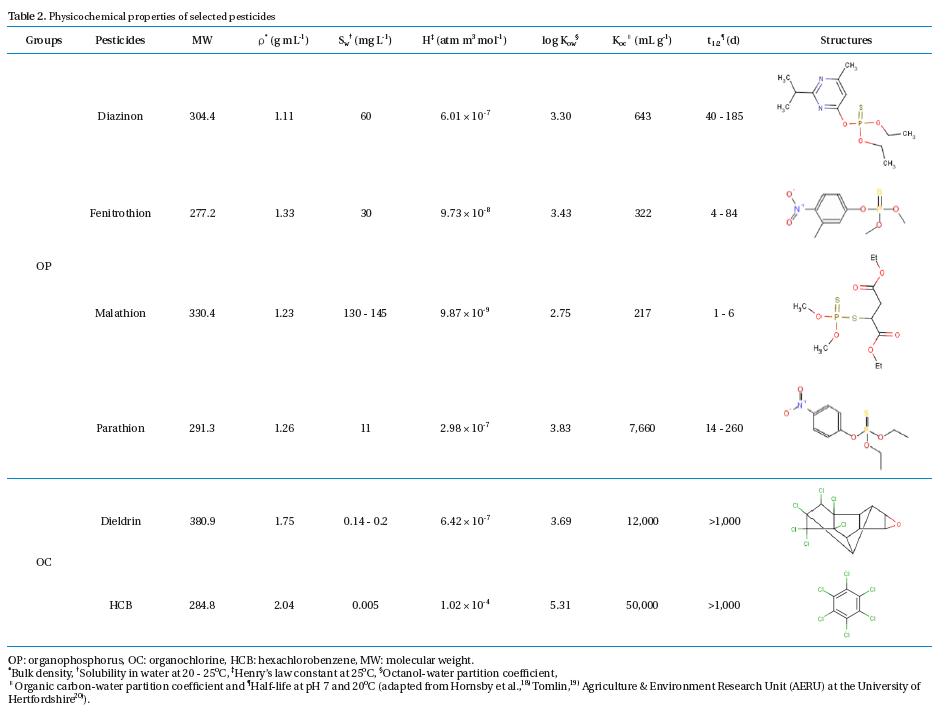
Physicochemical properties of selected pesticides
(500 mL/each PR) through Whatman GF/C filters. Collected samples were dried at 105℃ for 1 h in an oven and weighed on an analytical balance (Ohaus Corp., Pine Brook, NJ, USA). They were subsequently extracted by L-L extraction. The extraction method was similar to that in section 2.5.2, but with n-hexane used instead of EA. Furthermore, after filtration, the remaining solutions were subjected to L-L extraction similar to that described in section 2.5.1.
2.5.4. Pesticide Analysis
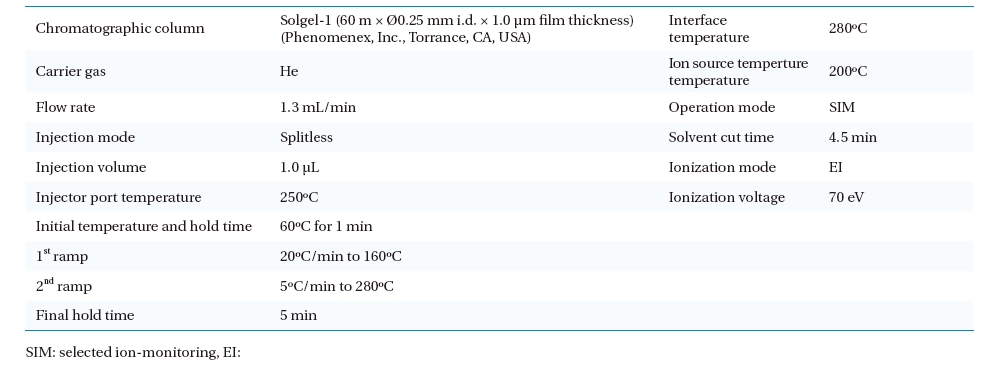
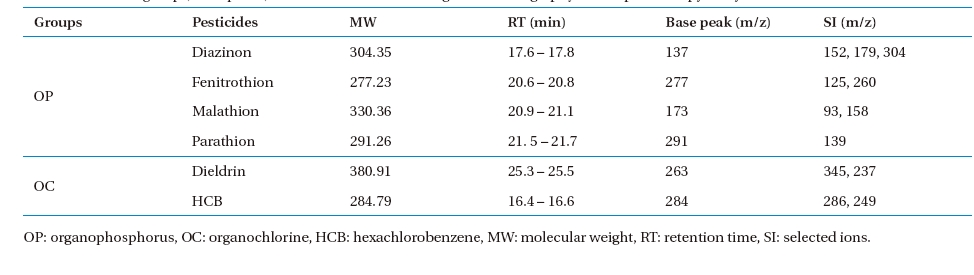
One microlitre of extracted solution was injected automatically into the GC/MS by an auto- injector/auto-sampler (AOC-20i; Shimadzu). The operational conditions of the GC/MS analysis are given in Table 3. A selected ion-monitoring (SIM) mode was chosen for the GC/MS operation. A SIM table was constructed for the GC/MS quantification. The most abundant ion as a base peak (target ion), retention time, and other ions for confirmation (selected ions) were obtained from a standard pesticide calibration (Table 4).
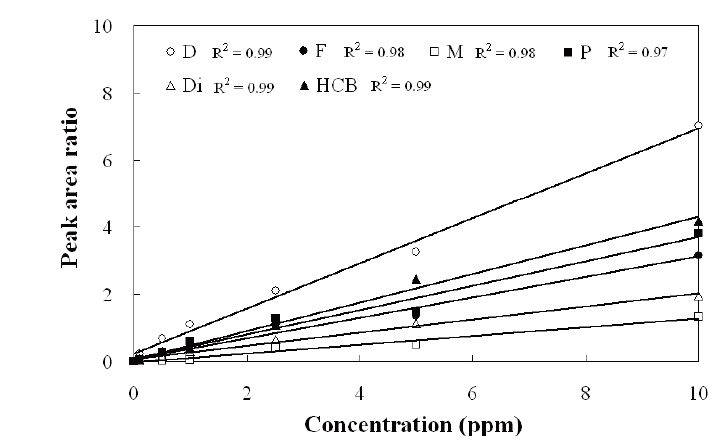
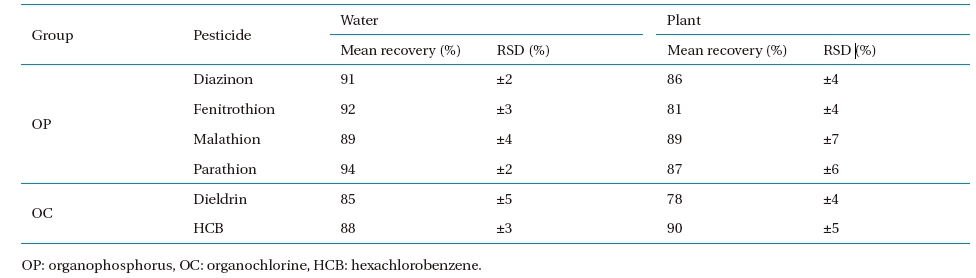
Standard curves were created for quantification, using standard solutions of the selected analytes in n-hexane and Endosulfan I-d4 as the internal analytical standard. Fig. 2 shows the linearity of the standard curves of each sample analyzed. The recovery study performed on blank samples (nutrient solution and plant samples with no pesticide contamination), spiked with known levels of the pesticide, is summarized in Table 5. Three replicates for each sample were carried out at three levels (25, 50, 100?μg?L?1 for water and 10, 50, 100?μg?kg?1 for plant, respectively), and the relevant recovery results, given as mean values, were 85 - 94% and 78 ? 90% for water and plant, respectively. The RSD was 2 ? 5% and 4 ? 7% for water and plant, respectively.
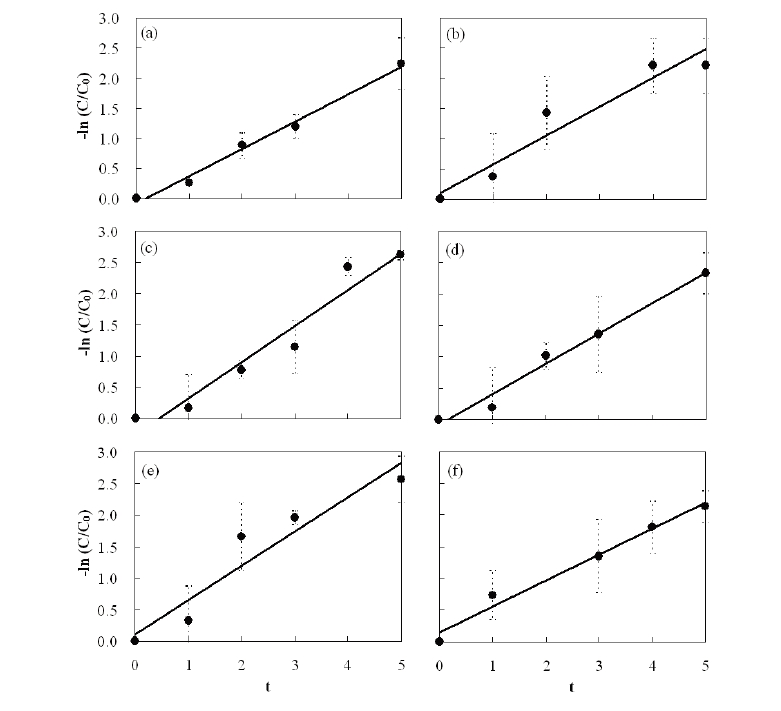
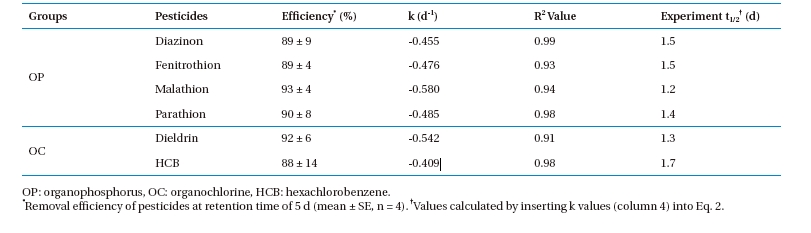
3.1. Kinetics of Pesticide Removals
The change in OP and OC concentrations in the PRs operated by the batch mode are described by first-order reaction kinetics (Fig. 3) and mathematically expressed as:
where C is the pesticide concentration (mg/L) in the PRs at time
Data obtained during the present investigation revealed that all experimental sets containing aquatic plants removed a substantial amount of pesticide. Approximately 88 ? 93% of the OP and OC pesticides in the water were removed by planted PRs at a retention
[Table 3.] Parameters for operating gas chromatography and mass spectroscopy
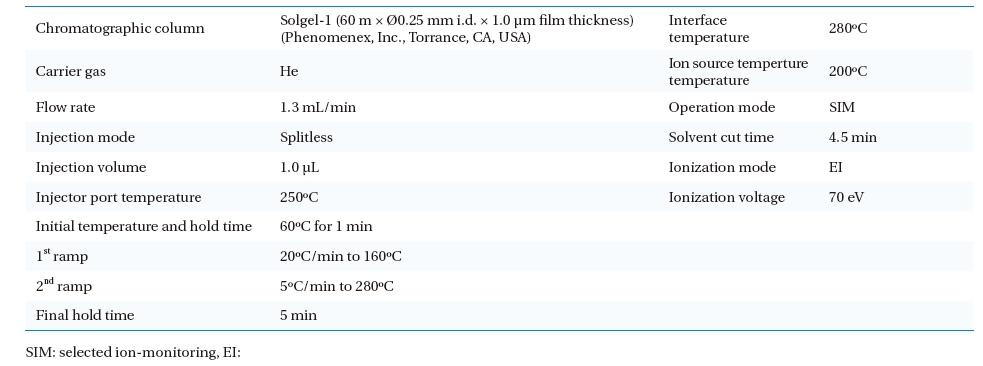
Parameters for operating gas chromatography and mass spectroscopy
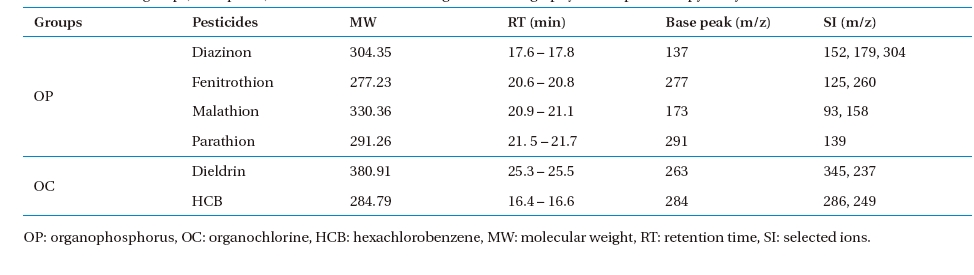
Chemical groups, base peaks, and selected ions used for gas chromatography/mass spectroscopy analysis
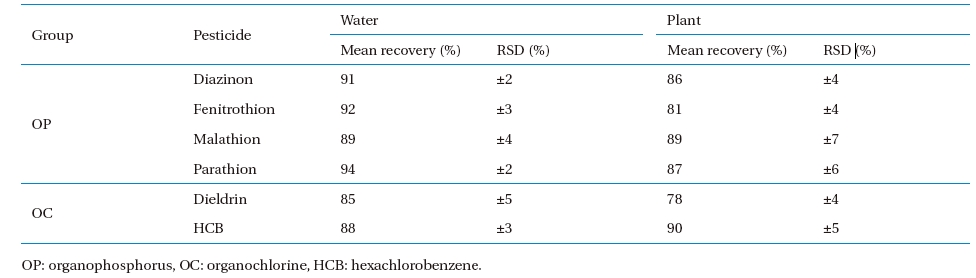
Average recoveries and relative standard deviations (RSDs, %) for OP and OC pesticides in water and plants fortified at 25, 50, and 500 (μg?L?1) in water and 10, 50, and 100 (μg g?1) in plant
time (RT) of 5 d. The reduction rate of the pesticides in the PRs fit Eq. 1 (R2 value >;0.9 for all pesticides). The
Estimates of the experimental
3.2. Plant Uptake of Pesticides
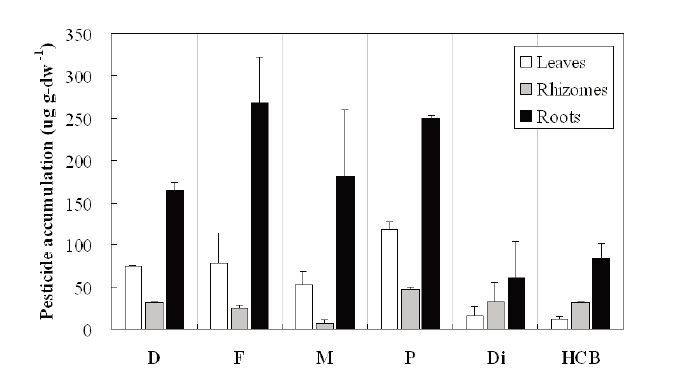
The weight-based pesticide accumulation in the plant organs of
[Table 6.] Kinetic parameters of OP and OC pesticide removal from aqueous medium
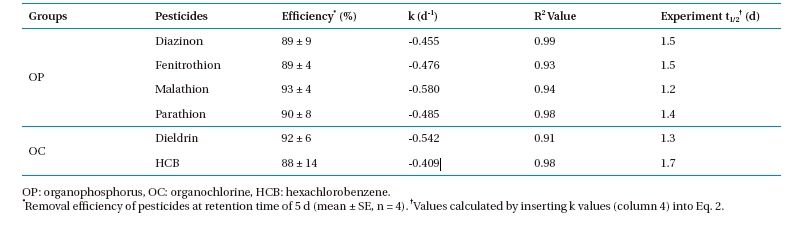
Kinetic parameters of OP and OC pesticide removal from aqueous medium
more than the OC pesticides. The highest accumulation of both OPs and OCs was found in the roots. The leaves of the
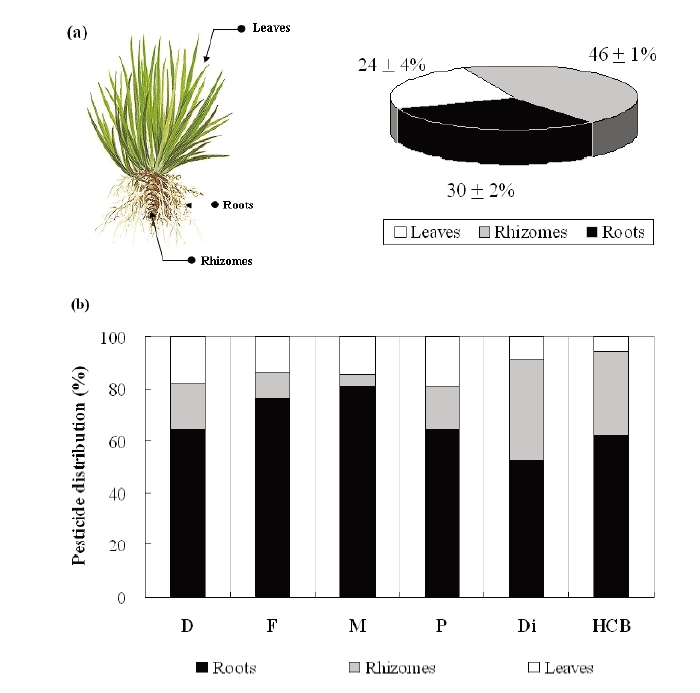
In addition, the overall distribution of pesticides based on plant biomass proportion is depicted in Fig. 5. According to the physiology of the plant,
Furthermore, the weight-based pesticide accumulation rates in terms of biomass wet- weight were estimated by dividing the total amount of pesticide accumulated in the plant with the total biomass (wet-weight) and treatment time. Estimates of weight- based pesticide accumulation rates of OP pesticides were 6,725 μg kgwet plant-1 d-1 for diazinon, 9,557 μg kgwet plant-1 d-1 for fenitrothion, 6,488 μg kgwet plant-1 d-1 for malathion, and 9,084 μg kgwet plant-1 d-1 for parathion. Estimates of weight- based pesticide accumulation rates of OC pesticides were 4,157 μg kgwet plant-1 d-1 for dieldrin and 4,912 μg kgwet plant-1 d-1 for HCB.
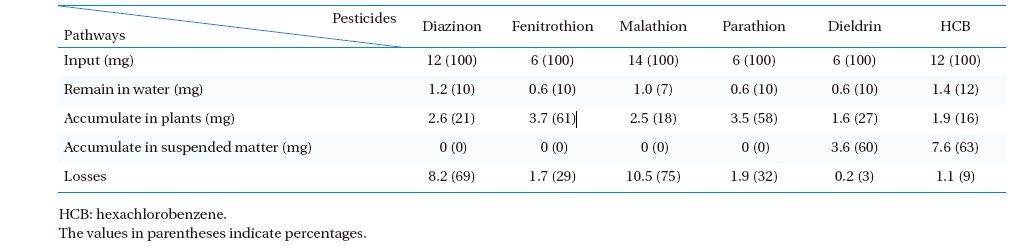
A mass balance of pesticides was performed on the PRs in order to determine pesticide removal pathways. For a batch mode of operation, the input amount of pesticides in the PRs must balance with the summation of total pesticides presented in the effluent treated water accumulated by plants and suspended matter (SM). However, all pesticides tested were organic substances likely to be degraded into other chemical breakdown-products as a function of time. The term “loss” is therefore inserted into the balance equation. A mass balance equation for distribution of a pesticide can be written as follows:
Total pesticides input to the PRs = Pesticides remaining in the water after treatment + Pesticides accumulated in plants + Pesticides accumulated in SM + Loss
[Table 7.] Mass balances of pesticides in phytoremediation process
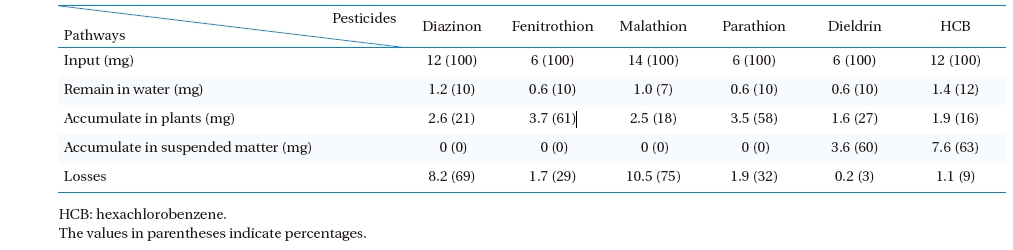
Mass balances of pesticides in phytoremediation process
The results of the mass balance are shown in Table 7. The amount of pesticides removed from the water was much higher than that accumulated by plants in the PRs. After treatment (RT = 5 d), the residual pesticides in the water were within a similar range of about 7 - 12% of its initial concentrations, whereas the accumulation of pesticides in
3.4. Plant Tolerance and Physical Toxicity Observation
In the first batch tests, all the experimental plants showed a slight reduction in plant growth, branching, leaf size, and root system. The mean biomass (wet-weight) after treatment increased only 0.8% in comparison with the original plant weight prior to treatment. The top portion of the leaves began to show yellow and dried, with portions of the roots beginning to show abnormal darkening and weakness. However, the plants seemed to acclimatize and endure the toxicity of the pesticides in the second batch tests. The dark green of the leaves was enhanced and observed in several experimental plants. The mean biomass increased 11.1% at the end of treatment.
It appeared that
The presented experimental
These results indicate that the chemical stability of pesticides used in the tests played an important role in the removal mechanisms and pathways in the PRs. Three main factors involving pesticide stability in an aquatic environment were structural stability, volatilization, and solubility. Four OP pesticides selected for the tests had low to medium structural stability (typical half-lives in aquatic environment = 1 - 206 d), as well as a high water solubility (Sw = 11 - 145 mg/L at 20 - 25℃) (Table 2). However, two OC pesticides selected for the tests were extremely stable (typical half- lives in aquatic environment >;1,000 d) and possessed an extremely low water solubility (0.005 - 0.2 mg/L) at 20- 25℃. Obviously, the distinction of pesticide removal pathways between OPs and OCs in the PRs was the amount of pesticides sorbed by the SM. All OC pesticides were found primarily in the SM as 60 - 63% of the total pesticide input to the PRs, whereas there was no sorption phenomenon of the OP pesticides by the SM due to the large differences of the Sw and organic carbon-water partition coefficient (Koc) between OP and OC pesticides (Table 2). Considering the OP and OC concentrations used in the tests (Table 1), all OP pesticides were very soluble in water and tended to not accumulate in the SM because of their strong polar nature. Contrary to the OC pesticides, both dieldrin and HCB were highly insoluble in water and much heavier than water (bulk density = 1.75 and 2.04 g mL-1), with a relatively high Koc (12,000 and 50,000 mL g-1). Thus, they could easily bind together with settled SM. This phenomenon was similar to the behavior of 17 OPs and 18 OCs in the Jiulong River of China reported by Zhang et al.2) where SM did not bear an obvious correlation with OPs due to their higher water solubility and weaker sorption capacity relative to OCs. Regardless, the required
Among the pesticides used in the tests, the OPs possessed high portions of unknown loss described by mass balances (Table 7). Loss of pesticides
The other causes of loss might arise from their chemical structures that typically undergo rapid environmental degradation, when compared with OCs,23) and possibly also plant metabolic conversion or phytotransformation.14) However, only the parent pesticide compounds were analyzed herein, not the breakdown products. Thus, further information regarding chemical intermediates was unavailable.
Malathion had the largest quantity of unknown loss in mass balance (75% of total malathion input into the PRs) due to its very small
Uptake and distribution of pesticides in plants were greatly influenced by pesticide species and plant physiology itself. The accumulation (in percent) of OP pesticides by
All pesticides accumulated and stored mainly in the plant below ground, especially in the roots (Figs. 4 and 5). This result was similar to the work reported by Bouldin et al.29) in that the roots of two aquatic plants,
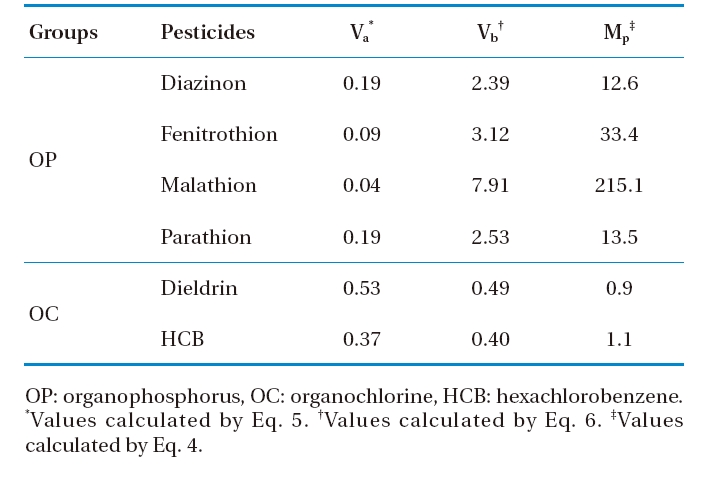
The usage of pesticide-tissue concentrations as a criterion for identifying plant-pesticide uptake could not indicate the mobile-capacity of pesticides in plants. The term “migration factor” (
[Table 8.] Migration factor of selected pesticides in plant
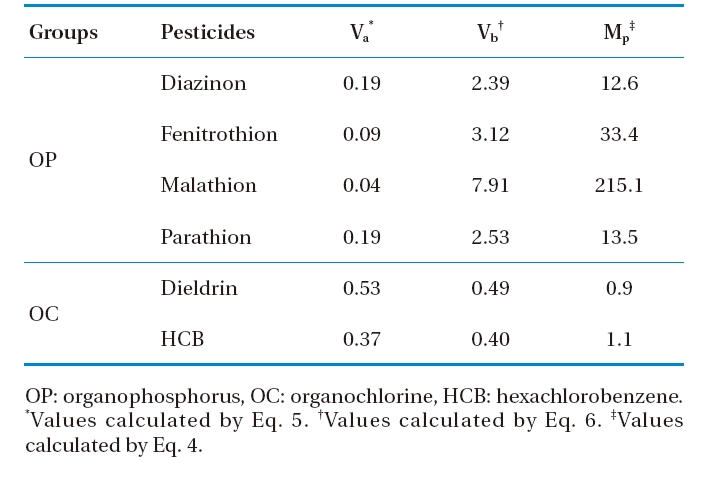
Migration factor of selected pesticides in plant
The
This study examined the detailed kinetics of six organic pesticides (diazinon, fenitrothion, malathion, parathion, dieldrin, HCB), along with the behavior of plant uptake, information on pesticide/plant partition coefficients, and removal mechanisms/pathways in PRs grown with
According to mass balance analysis, the chemical stability of the pesticides, especially diverse molecular structures, solubility, and typical half-lives in an aquatic environment, clearly influenced the removal pathways in the PRs. The main distinction of the removal pathways between OP and OC pesticides in the PR treatment were the plant accumulation and sorption by SM. The plant accumulation of the pesticides varied broadly (16 ? 61% of total pesticide input to PR) depending on the pesticide species. The highest accumulation rate of pesticides occurred with malathion (about 9084 μg kgwet plant-1 d-1), while the lowest was with dieldrin (about 4157 μg kgwet plant-1 d-1). All OPs had no sorption in the SM, while nearly 60 - 63% of the total pesticides input of the PR were accumulated by the SM. High portions of unknown loss were found in the mass balances of diazinon and malathion. This could be explained by the very small half-lives of the two pesticides in an aquatic environment.
Distribution of pesticides in