The water quality in eutrophic lakes is affected by serious problems, such as explosive increases in Cyanobacteria. Some kinds of Cyanobacteria produce metabolites of musty substances and toxins in their cells. Damage to human health as a result of microcystin, one of the toxic, has been reported in many countries.1)-3) Therefore, it is necessary to remove the Cyanobacteria and nutrient salt from eutrophic lake water to improve the water quality. The pH of the water at the surface of many lakes is higher than pH 9 because of frequent algae blooms, which inhibits the coagulation of traditional coagulants.4)
The removal efficiencies of phytoplankton, ammonia nitrogen, nitrate nitrogen and phosphoric acid are discussed with respect to the hybrid technique: (a) chemical compounds, i.e. the formation of magnesium ammonium phosphate and magnesium hydroxide precipitation, and (b) the electrostatic bridging effect between micro-bubbles and phytoplankton.
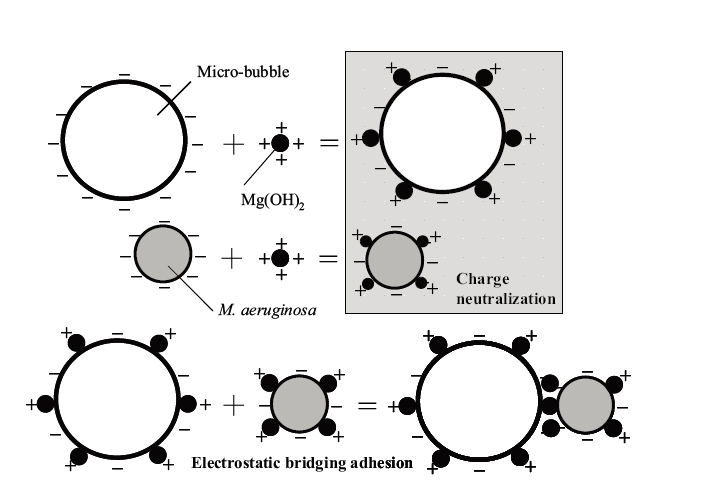
1.1.1. Electrostatic bridge effect
With the electrostatic bridging effect of a micro-bubble and phytoplankton, the Van der Waals and electrostatic forces between the substances are important factors. The electrostatic bridging effect of a micro-bubbles and phytoplankton is shown schematically in Fig.1. Micro-bubbles and phytoplankton are attached to magnesium hydroxide precipitates on their surfaces, which then approach the iso-electric point. Collisions between micro-bubbles and phytoplankton can be facilitated by charge neutralization. Consequently, electrostatic uniting constructs a bridge with the magnesium hydroxide precipitate, which allows micro-bubbles and phytoplankton to be adsorbed. Therefore, phytoplankton adsorbed onto the micro- bubbles are carried to the water surface with the micro-bubbles.
1.1.2. Magnesium compounds
The mechanisms are as follows: Firstly, the positively charged magnesium hydroxide precipitate is formed at pH values higher than 9, which is then adsorbed onto the surfaces of the phytoplankton and micro-bubbles. Thus, phytoplankton attaches to the micro-bubble surface by uniting of an electrostatic bridging effect, and is then carried to the water surface. Secondly, magnesium ammonium phosphate (MAP, MgMH4PO46H2O) and magnesium phosphate precipitates (Mg3(PO4)24H2O) are formed by the reaction of magnesium, phosphate and ammonium ions at high pH values. It is then anticipated that the phosphate and ammonium ions will be removed from eutrophic water. The positively charged magnesium hydroxide precipitate is firstly formed at pH values higher than 9,5), 6) and is adsorbed onto the surface of phytoplankton and micro-bubbles. Thus, phytoplankton is attached to the surface of a micro-bubble by an electrostatic bridging effect, and is then carried to the water surface. Secondly, magnesium ammonium phosphate (MAP), including phosphoric acid and ammonium ions, is formed at pH values higher than 5 to 7, as shown in Eq.1.7), 8) It has been reported that the formation of MAP crystals depends on the chemical species of phosphoric acid and ammonia present with variations in pH, and the generation of the magnesium phosphate progresses at pH values higher than 9, as shown in Eq.1 and 2. Therefore, it is suggested that phosphoric acid and ammonia can be removed by controlling the pH.
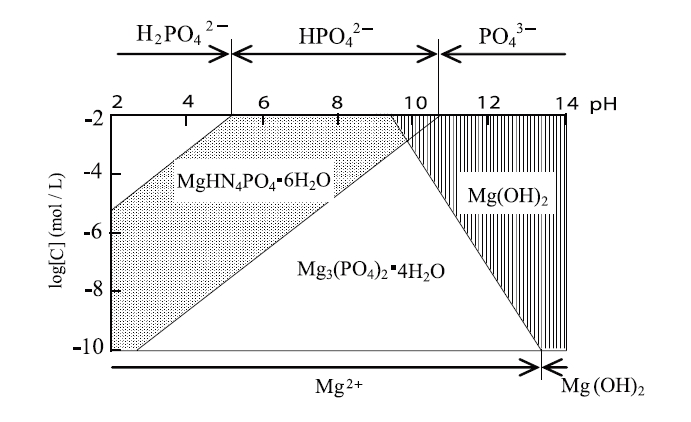
In general, MAP crystals are formed under pH conditions >;5-7. However, from our observations, these magnesium compounds that include phosphoric acid depend on the formation of phosphoric acid ions with variations in pH. On the other hand, the magnesium hydroxide precipitate is the main species under pH conditions >;9-10, which will be formed by the hydrolysis reaction shown in Eqs 3 and 4 5)
The variations in dissolved phosphate and magnesium ion species as a function of the pH are shown in Fig.2. Under neutral pH conditions, the hydrogen phosphate ion will be the main species, where MAP is formed. In the second region, i.e. under higher pH conditions, the concentration of dissolved magnesium ions will decrease with the formation of a magnesium hydroxide precipitate, and the phosphoric acid ion will be the main species, with the simultaneous formation of magnesium phosphate. Therefore, phosphate and ammonia are suggested to be removed in the under neutral pH conditions. On the contrary, it is expected that the removal efficiency of ammonia will decrease with the formation of a magnesium hydroxide precipitate.
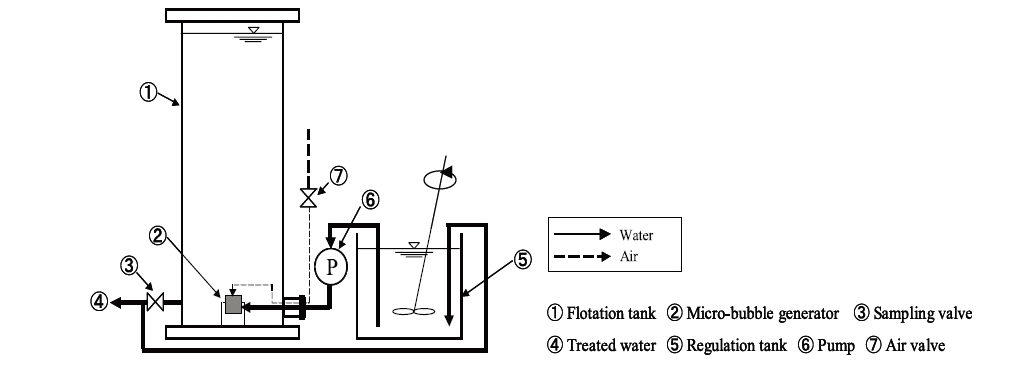
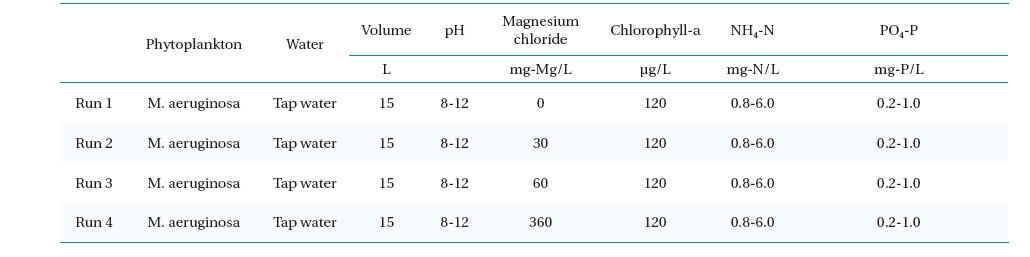
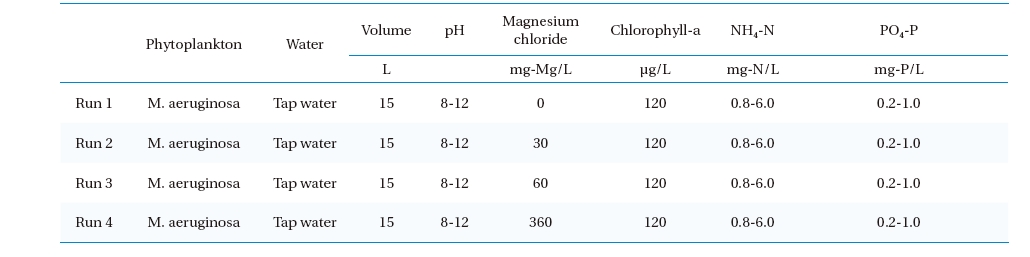
Microcystis was used as the Cyanobacteria in this study, and were bred in artificial raw water, with the chlorophyll-a concentration maintained at approximately 120μg/L in each experiments. The NH4-N and PO4-P concentrations were adjusted to 0.8 or 6 mg-N/L and 0.2 or 1 mg-P/L respectively; similar quality conditions as those found in eutrophic water. The pH conditions of the raw water was maintained within the range 8∼12 as shown in Table 1. The flotation experiment procedure was as follows: First, a
The removal efficiency of M. aeruginosa was calculated from the chlorophyll-a concentration using Eq.5. To evaluate froth separation, the removal rate due to flotation was calculated from the thicknesses of the froth and sedimentation layers using Eqs 6 and 7.
Where R is the removal efficiency of chlorophyll-a (%), M(F) the weight of froth (mg), R(F) the flotation efficiency (%), A the final concentration of chlorophyll-a at t = 90min (mg/L), B the initial concentration of chlorophyll-a at t=0min (mg/L), C the thickness of the froth layer (mm), D the thickness of the sedimentation layer (mm) and W the volume of M. aeruginosa suspension (= 60L).
[Table 1.] Experimental conditions
Experimental conditions
3. Experimental Results and Discussion
3.1. Adhesion of M.aeruginosa and Coagulant Materials
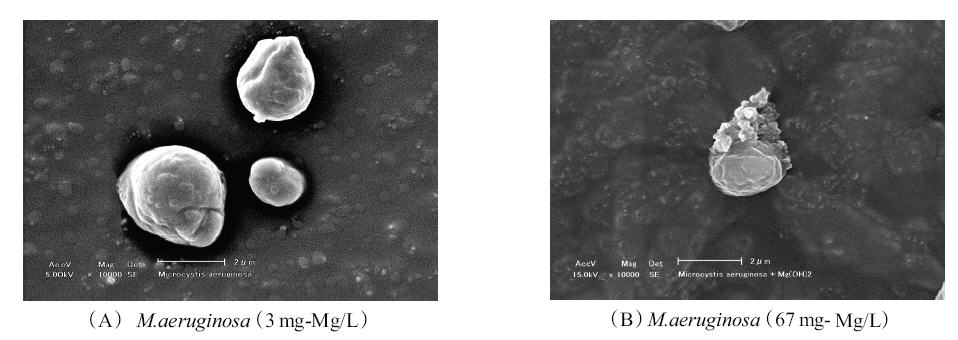
Generally, it is known that particles; M.aeruginosa and micro-bubbles, do not adhere to each other in water, as their surfaces are both covered by negative charges. Photo. 1., taken by SEM, shows the surface of M.aeruginosa at two different magnesium concentrations, 3 and 67 mg/L. Crystal magnesium hydroxide can be observed on the surface of the M.aeruginosa particle in Photo. 1. (B).
3.2. Removal efficiency of Microcystis particles
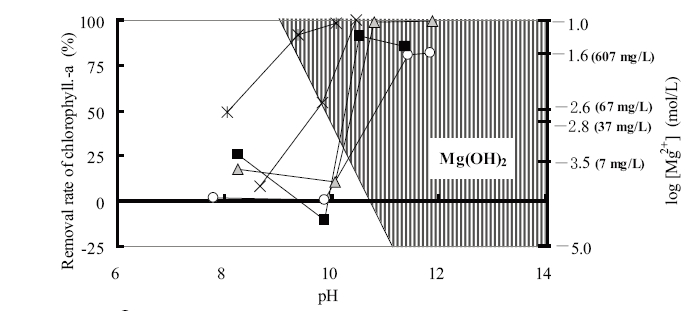
The change in the removal rate of chlorophyll-a with respect to the magnesium chloride dosages and pH values is shown in Fig.4. Here, the shaded area is designated as the region of magnesium hydroxide settling. On the addition of magnesium chloride, removal efficiency of chlorophyll-a tended to increase with increasing pH in accordance with the formation of the magnesium hydroxide precipitate. A rapid increase in the removal rate of chlorophyll-a was observed within the pH range 10-11, with the maximum removal rates of 91-99% observed at pH 10.5 and 10.8 with 30 and 60mg-Mg/L, respectively. Here, the chlorophyll-a concentration decreased from 120 to 20-1μg/L and the rate of chlorophyll-a collection was also very high.9)
When magnesium chloride was used as a coagulant, a high removal efficiency of chlorophyll-a was obtained in accordance with the formation of a magnesium hydroxide precipitate. Therefore, it is possible to use magnesium chloride as a coagulant for the solid-liquid separation under high pH conditions.
3.3 Removal efficiency of ammonia nitrogen and phosphoric acid
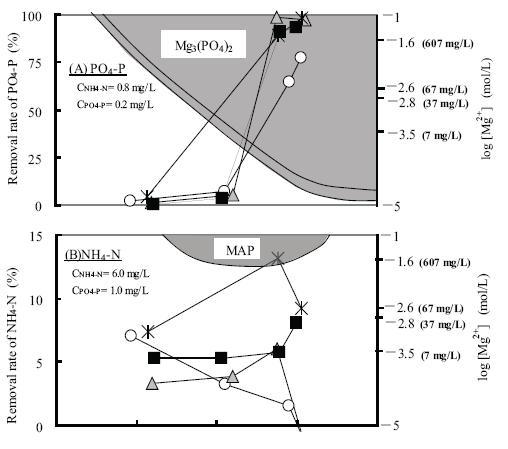
The removal efficiencies of NH4-N and PO4-P with magnesium chloride dosages are shown in Fig. 5. Here, the shaded part is designated as the region of MAP and magnesium phosphate formation. Firstly, in accordance with the formation of magnesium phosphate, the removal efficiency of phosphoric acid increased with increasing pH. Here, the phosphoric acid ion concentration decreased from 0.47 to 0.02 mg-P/L (pH>;10.5). Secondly, the removal efficiency of ammonia was found to be a constant 5-7% in the pH range 8∼12. Therefore, with respect to a magnesium chloride dose of 600mg-Mg/L, with high phosphoric acid and ammonia concentrations, the observed removal efficiency of ammonia was 13.7% in accordance with the formation of MAP.
Therefore, the results indicate that it is possible to remove phosphoric acid and ammonia from water; the removal of phosphoric acid from water, especially, was expected under high pH conditions.
3.4. Removal characteristic caused by magnesiumhydroxide
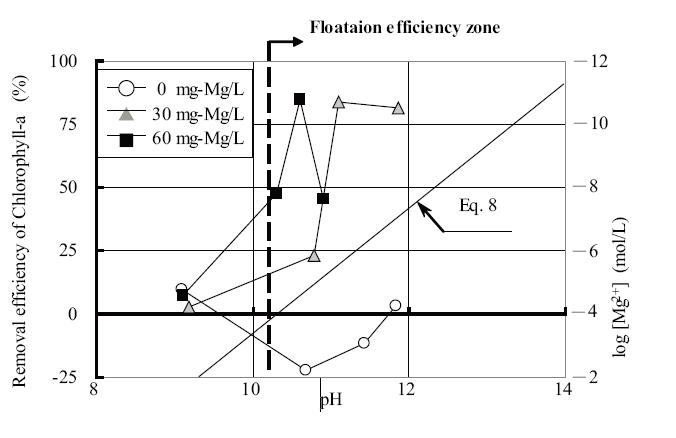
The changes in dissolved magnesium ions and removal efficiency of chlorophyll-a with respect to magnesium chloride dosages and pH are shown in Fig. 6; the percentages shown in this figure refer to the flotation efficiency, and was calculated from Eqs 6 and 7. The dotted line shows the dissolved magnesium ions calculated from Eq.8.
Where K is the solubility product on Mg(OH)2 (K=5.61×10-12) and Kw the solubility product of water (Kw=10-14). Decreases in the dissolved magnesium ion concentration resulted from the generation of a magnesium hydroxide precipitate, which increased with increasing pH.
On the addition of magnesium chloride, the removal efficiency of chlorophyll-a tended to increase with increasing pH. The removal efficiency of chlorophyll-a was observed to rapidly increase within the pH range 10∼11. The pH values changed with coagulant dosages. The chlorophyll-a concentration decreased from about 150 to 20μg/L. The maximum removal efficiency of chlorophyll-a was observed to be 84% at pH 10.6. Therefore, the results indicate it is possible to use magnesium chloride as a coagulant for the solid-liquid separation without regulating the pH and to obtain a natural high removal efficiency under high pH conditions.
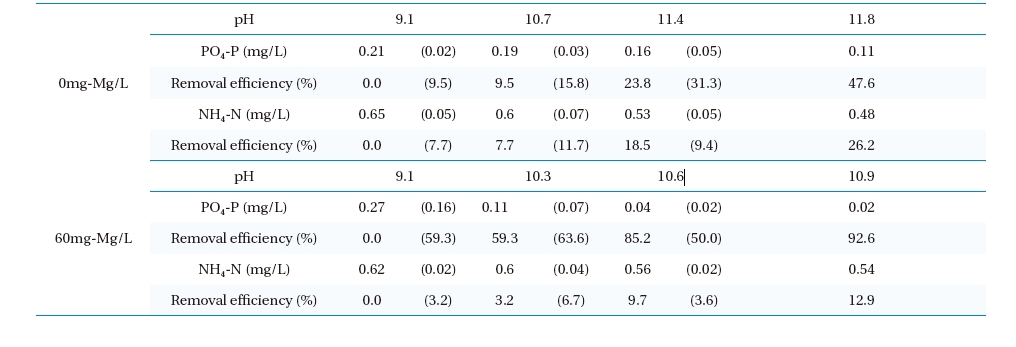
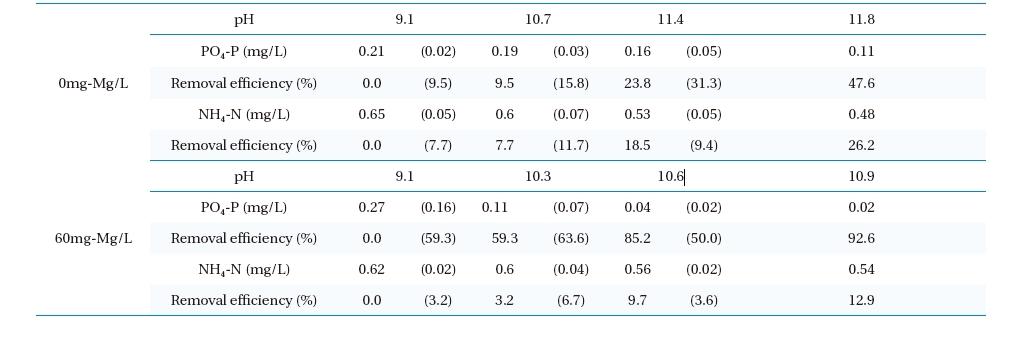
In the high pH zone, the experimental results for the removal efficiencies for NH4-N and PO4-P with magnesium chloride dosage are shown in Table 2. The removal efficiencies and the differences in the NH4-N and PO4-P concentrations with respect to pH from the start to finish of the experimental period are shown in parentheses. The removal efficiency of PO4-P increased with increasing pH. With a magnesium chloride dose of 60mg-Mg/L, the NH4-N concentration decreased from 0.62 to 0.54mg-N/L (13%), and that of PO4-P decreased from 0.27 to 0.02mg-P/L (92%). The maximum removal efficiencies and decreases in the NH4-N and PO4-P concentrations before and after the change in pH were observed to be 6.7% (0.04mg-P/L) and 63.6% (0.07mg-N/L), respectively. This also infers that the decreases in the NH4-N and PO4-P molarities were 6×10-6 and 8×10-6 mol, respectively, and implied the formation of MAP. On the other hand, without the addition of magnesium chloride, the magnesium ions included in the
[Table 2.] Removal efficiencies of ammonia nitrogen and phosphoric acid
Removal efficiencies of ammonia nitrogen and phosphoric acid
This study examined the possibility of using a flotation system combining a hybrid technique (chemical compounds and electrostatic bridge) for application to raw water with a high pH containing phytoplankton, as well as the zeta potential value of the phytoplankton surface and the removal efficiency of phytoplankton, ammonia, nitrogen, and phosphoric acid. The results obtained from various experiments were as follows:
1. The flotation system using a hybrid technique (chemical 111compounds and electrostatic bridge) can be applied to raw water with a high pH containing phytoplankton. The maximum removal efficiencies of phytoplankton, ammonia, nitrogen and phosphoric acid were also estimated
2. The chlorophyll-a concentration decreased from about 150 to 22220μg/L, with a maximum removal efficiency of 84% at pH 10.5.
3. It is possible to remove NH3334-N and PO4-P from eutrophic water. The concentrations of NH4-N and PO4-P decreased from 0.62 to 0.54mg-N/L (13%) and from 0.27 to 0.02mg-P/L (92%), respectively. the optimal pH conditions for the maximum removal of chlorophyll-a were also obtained; the removal efficiencies and amounts of NH4-N and PO4-P removed before and after the change in pH were observed to be 6.7% (0.04mg-P/L) and 63.6% (0.07mg-N/L), respectively.
4. M.aeruginosa particles were observed to be charge neutral on the adhesion of magnesium hydroxide precipitate in the pH range 10.5 to 11.
In the field of water basin management, this hybrid system combining magnesium compounds and an air flotation technique will be an efficient system as a water quality purification technology.