Cement manufacturing is an energy- and resource-intensive process requiring large amounts of raw materials and fuel. A cement plant consumes from 3,000 to 6,500 MJ of fuel per ton of clinker produced (electricity and transportation together add about 10% more to the total energy) depending on the raw materials and the process used.1) Cement production involves the burning of the intermediate clinker product in a kiln at higher temperature. The process requires temperatures of 2,000℃ in the main burner, 1,450℃ for the materials to make clinker, and 1,000℃?1,200℃ in the pre-calciner.
In order to reduce the high-cost energy requirements for the decomposition of waste and by-products with high calorific value can be used as fuel in cement kilns (CKs). Some waste and by-products containing useful minerals such as calcium, silica, alumina, and iron can be used as raw materials in the kiln.2) Cement kilns offer the easiest option for destruction of unwanted organic wastes due to their high incineration temperature, large furnace area, longer residence time, and the alkaline environment inside the kiln.3) Burning of waste in CKs has various benefits such as destruction of waste from other industries, energy recovery, conservation of non-renewable resources and reduction of cement production cost.4,5) Wastes have long been used as alternative fuels and raw materials in CKs in Europe, Japan, USA, Canada, and Australia, and more recently in Korea. Fuel substitution in the European Union (EU) cement plants use an average of 12% wastes as alternative fuels and raw materials.6) The cement industry in Korea began burning waste from used tires since 1995, following which CKs were recognized as waste disposal facilities.7) The CKs in Korea use coal as the primary fuel also use waste as an auxiliary fuel to economize production.8) After a shift in waste management policy from landfill to reduction, recycling and safe and sanitary treatment of wastes have been a priority in Korea, and large amount of combustible waste has been burned in incinerators and CKs.
Cement manufacturing causes environmental impacts at all stages, including emissions of airborne pollution (dust, gases), noise and vibrations, and damage to the countryside from quarrying. Various initiatives have been taken in the EU and USA to regulate the emission of hazardous air pollutants (HAPs) from CKs. The co-incineration of waste (hazardous and non-hazardous) in CKs is encouraged in the EU, and is currently regulated by the EU Directive2000/76/EC.9) U.S. EPA regulates HAPs from CKs by maximum achievable control technology regulation (MACT). In addition, CKs burning hazardous waste are regulated separately.10,11) The Asia-Pacific partnership (a government program including the cement industry in the USA, Japan, China, South Korea, Australia, and India) is working together on various issues, including emission management in the cement industry.12)
In spite of worldwide efforts to reduce emissions from CKs, there exist limited literatures on HAP emissions from CKs burning wastes. This paper summarizes the HAP emission characteristics, transformation within the system, and the control efficiency achieved by the existing air pollution control devices (APCDs) in CKs co- burning waste. In addition, heavy metal speciation in cement kiln dust (CKD) and its potential fate in the environment are discussed.
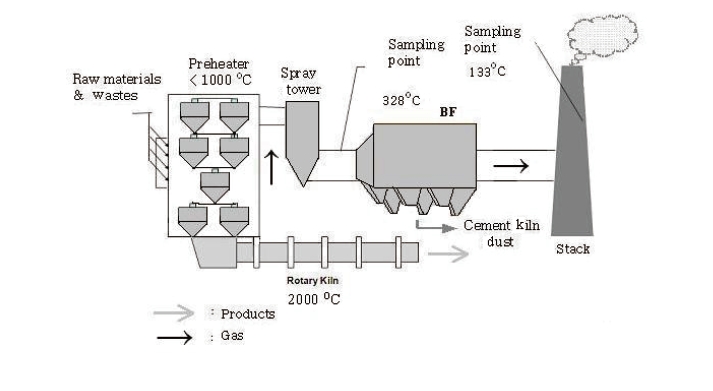
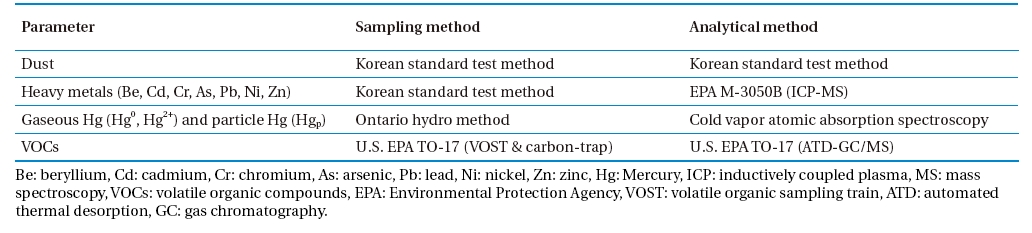
Emission tests were carried out at three commercially-operating CKs co-burning waste as an auxiliary fuel. CKs tested consisted of a rotary kiln, spray tower, and bag filter (BF) arranged in a series for flue gas cleaning. The APCDs configuration is shown in Fig. 1. Dust, heavy metals (beryllium [Be], cadmium [Cd], chromium [Cr], arsenic [As], lead [Pb], nickel [Ni], zinc [Zn], mercury [Hg], and Hg speciation) and volatile organic compounds (VOCs) were measured. All parameters were sampled and analyzed before the BF and stack of each facility. Dust sampling and analysis were carried out according to the Korean standard test method. Heavy metals were sampled using the Korean standard test method and analyzed by U.S. EPA M-3050B, inductively coupled plasma-mass spectroscopy (ICP-MS), method.
Mercury and its speciation were sampled and analyzed by the Ontario hydro method. Isokinetic samplings were performed, and the flue gas withdrawn was passed through the filtration system followed by a series of impingers in an ice bath. Hgp was collected on the filter; Hg2+ was collected in three impingers with 1 N potassium chloride solution; and Hg0 was collected in an impinger containing 5% nitric acid and 10% peroxide solution and in three impingers containing a solution of 10% sulfuric acid and 4% potassium permanganate. Any remaining moisture was removed in the last impinger in a series containing silica gel. Samples were recovered, digested and analyzed using cold vapor atomic absorption spectroscopy Hg analyzer (RA915+, Lumex). Volatile organic compounds were sampled by U.S. EPA TO-17 (volatile organic sampling train [VOST] and carbon trap) and analyzed by U.S. EPA TO-17 (automated thermal desorption gas chromatography/mass spectrometry [ATD GC/MS]) method. Sampling and analysis methods are summarized in Table 1. All sampling tests and analyses were carried out several times at the same point to ensure their reliability.
3.1. Raw Materials, Fuel and Waste
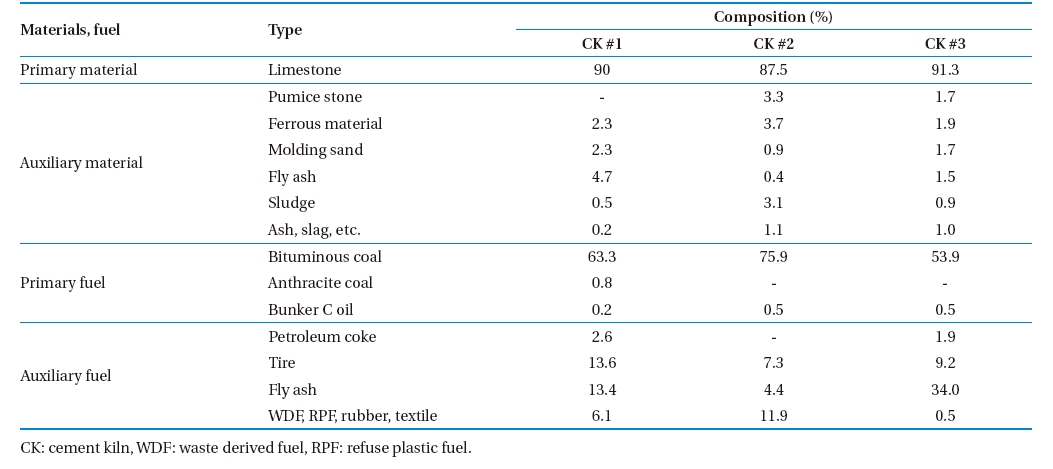
In addition to primary fuel-coal and oil; auxiliary fuel burned in the examined CKs included petroleum coke, fly ash, waste derived fuel (WDF), refuse plastic fuel (RPF), tire, rubber and textile. Ferrous material, pumice stone, molding sand, fly ash, sludge, and slag were used as auxiliary materials. Gypsum from desulphurization and slag mixture were also used during the clinker production process. The composition of major raw materials, fuel, and waste used in the CKs is shown in Table 2. Coal and oil were fed at the rotary kiln, and waste was fed at the preheater.
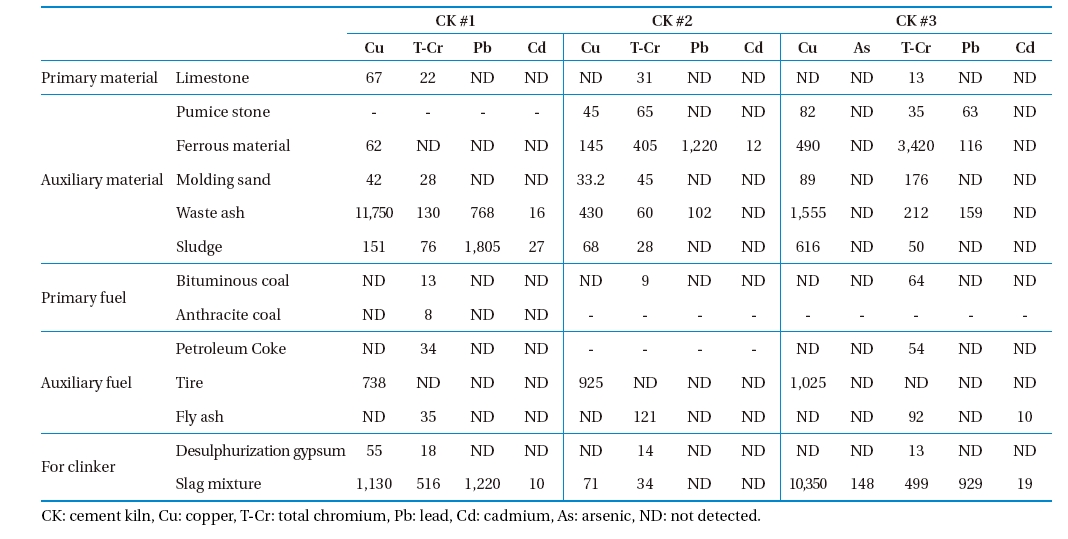
The measured concentrations of heavy metals in the raw material, fuel, and waste are shown in Table 3. As shown in the table, auxiliary materials, fuels contained a higher concentration of heavy metals than the primary materials and fuels did. Arsenic was not detected in any of the sample types tested in CK #1 and CK #2. In general, waste ash, sludge, and slag contained higher concentrations of heavy metals. The heavy metals concentrations in the waste vary between the CKs tested. For example, Pb was higher in the sludge and slag mixture in CK #1, in ferrous materials in CK #2, and in the slag mixture in CK #3. The input concentrations of metals vary according to variations in composition of the fuel, waste, auxiliary material, and auxiliary fuel.
3.2. Heavy Metals Emission (except Hg)
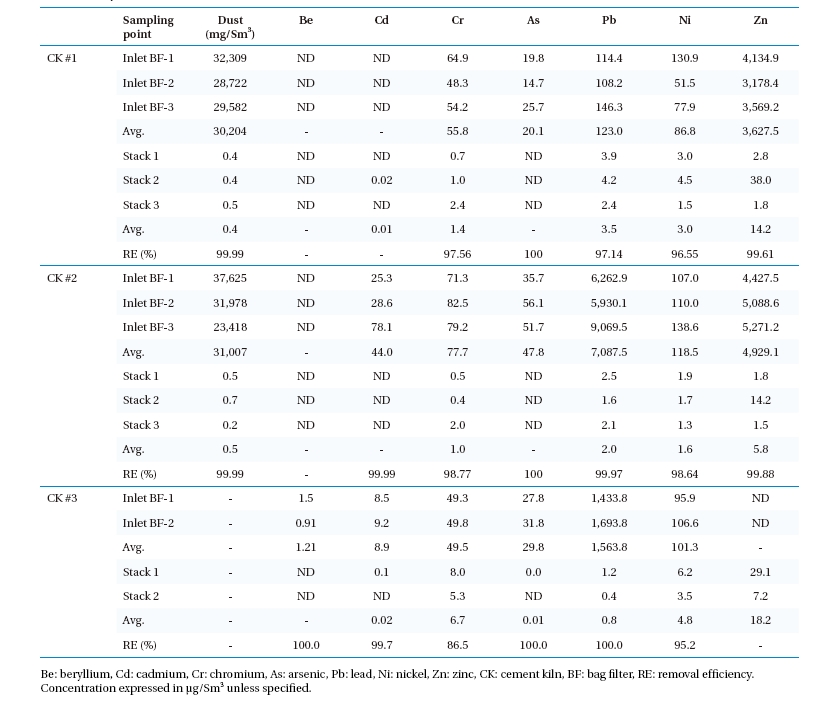
Heavy metals introduced in the CK may either incorporate in the cement clinker, be removed by APCDs, or emitted from the stack. Increasing heavy metal input by fuel and waste increases its content in cement and in the environment (directly by emission, or by leaching to ground water when CKD is land filled). On average, 0.45 mg/Sm3 of dust was emitted from the stack, and removal efficiency was above 99.99%, since it was filtered efficiently by BF. On average, removal of heavy metals (except Hg) efficiency?was above 98.5%; this was due to the operation of a high-efficiency BF. The bag filter had removal efficiencies in the range of 99?99.9% for most trace elements such as Pb, Cd, Ni, and manganese (Mn).13)
Heavy metal emission measurements before BF and stack are shown in Table 4. The measured concentration of heavy metals emitted from CK stacks ranged as follows: Zn 5.8 μg/Sm3 to 18.2 μg/Sm3, Ni 1.6 μg/Sm3 to 4.8 μg/Sm3, Cr 1.0 μg/Sm3 to 6.7 μg/Sm3, Pb 0.8 μg/Sm3 to 3.5 μg/Sm3, Cd 0.01 μg/Sm3 to 0.02 μg/Sm3, and As (CK#3: 0.01 μg/Sm3). The greatest emissions from CK #3 were Zn, Cr, Ni, Cd; from CK #1: Zn, Pb, Ni; and from CK #2: Pb. Zn enters a CK through lime, clay, and fuel. In addition, co-incineration of waste such as oil and tires can increase its concentration noticeably.14,15) Higher Zn emissions from CKs #1 and #3 may be
[Table 1.] Sampling and analytical methods
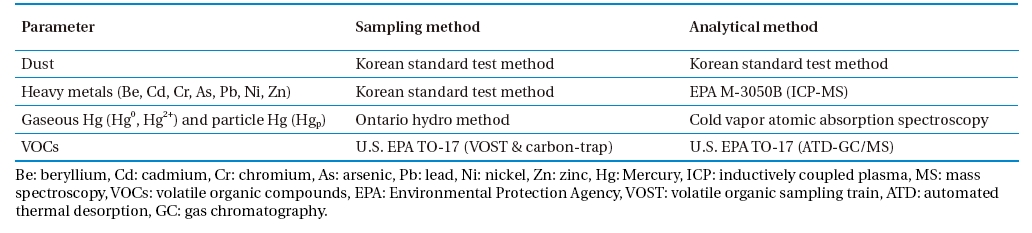
Sampling and analytical methods
[Table 2.] Composition of raw material, fuel, and waste
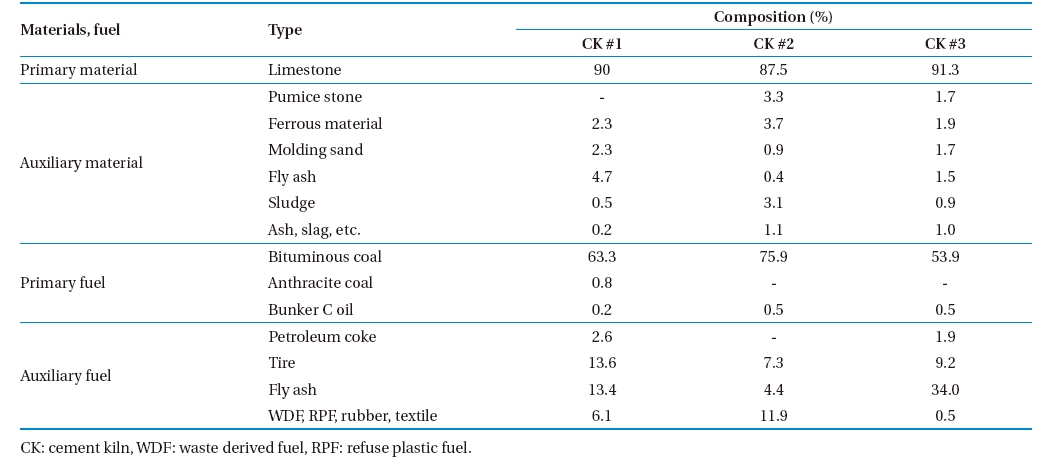
Composition of raw material, fuel, and waste
[Table 3.] Concentration of heavy metals in raw materials, fuels, and wastes (mg/kg)
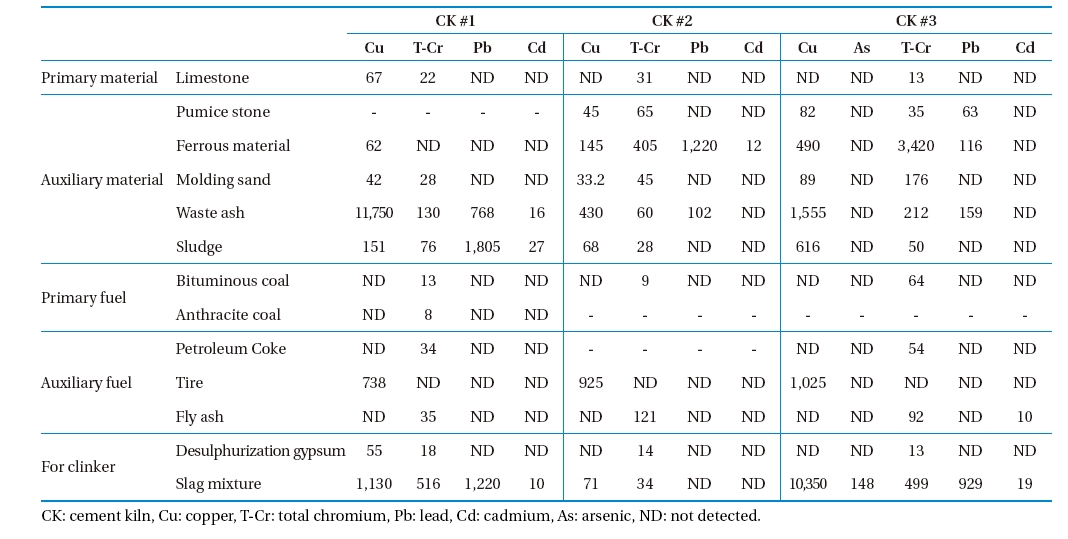
Concentration of heavy metals in raw materials, fuels, and wastes (mg/kg)
due to the higher portion of rubber and tires in the waste burned. Pb is mainly introduced into CKs by conventional fuels or burned waste.16) Carrasco et al.17) reported that when scrap tires are used as a fuel substitute in CKs, the rate of emission for metals increased 487% for Zn, 127% for Pb, and 339% for Cr. The German Cement Works Association (in German, Verein Deutscher Zementwerke)18) reported that As, Cd, Cr are associated with ironworks waste that are frequently burned in CK, while vanadium (V) and Ni are higher in oil coke. As was not detected in the raw materials and waste or in the flue gas emission of CKs #1 and #2, whereas As was detected in the slag mixture and in the flue gas of CK #3. Total-Cr (T-Cr) was present in all the raw materials, fuel and wastes tested (except tires). Cr was highest in wastes burned in CK #3, and in flue gas emissions from CK #3. Pb concentration was highest in waste (waste ash, sludge, and slag mixture) and its concentration in flue gas in CK #2. Since Cd had a low concentration in waste, its concentration in emission flue gas was correspondingly small. Thus, emission variations of metals in tested CKs were related to raw materials, fuel, and waste fed.
Distribution of metals within the CK varies depending on its boiling and melting temperatures. Trace elements during combustion have been classified into three classes.19) Class I elements do not volatilize during combustion, and are distributed in bottom ash and fly ashes. Class II elements vaporize but are found in fly ashes after condensation on particulates and by a nucleation mechanism due to decreasing temperature. A significant proportion of these fine particles are in the sub-micron size class where the duct control system is less effective. Class III elements vaporize and condense partly within the system.20) Among the measured heavy metals in this study, Hg is in Class III, easily passing through pollution control devices and speciating in the gaseous phase. Zn, Ni, Pb, Cd, Be, and Cr fall in class II and are
[Table 4.] Heavy metal and dust emissions from cement kilns
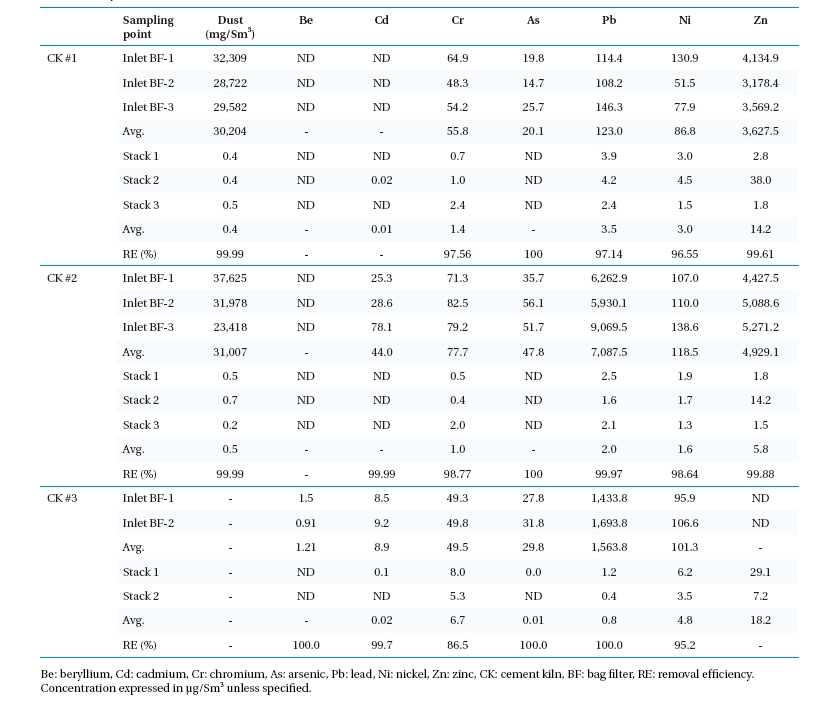
Heavy metal and dust emissions from cement kilns
mostly removed by pollution control devices. Earlier studies show that non-volatile metals entering CK process are mainly (55?70%) distributed in clinker,21-23) a minor portion is distributed in CKD, and a very small portion (0.1%) is emitted from stack.24) The major portion of semi-volatile metals are distributed in CKD (66?98%)25) less in clinker (34?2%) and the emissions were less than 0.75%.
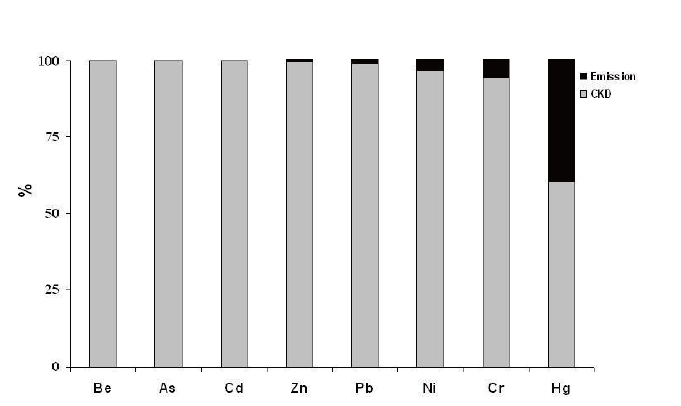
Heavy metals have also been classified according to toxicity.24,26) Class I includes highly toxic metals (Cd, Hg, titanium [Ti], and As), Class II includes metals of medium toxicity (Pb, cobalt [Co], Ni, selenium [Se], and tellurium [Te]) and Class III includes metals of low toxicity (Cr, copper [Cu], platinum [Pt], V, tin [Sn], palladium [Pd], Mn, and rhodium [Rh]). On average, 3.3% of the heavy metals of medium and low toxicity (Pb, Ni, Cr) entering BF were released into the atmosphere. Among the toxic heavy metals measured, 0.14% of Cd, 0.01% of As, and 40% of Hg entering the BF were released into the atmosphere.
3.3. Mercury Emission and Speciation
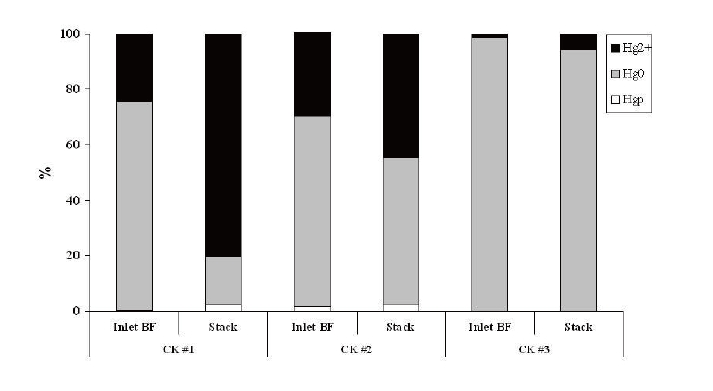
Mercury speciation into particulate, elemental, and oxidized form in the flue gas is important since the overall efficiency of the control system depends on speciation. Hg speciation, its removal within APCDs, and emission concentrations are affected by type of fuel or waste, operating conditions, and flue gas composition. In addition, the use of fly ash from coal-fired power plants in cement manufacturing as an additive increases Hg emission. On average, concentrations of Hg ranged from 13 μg/Sm3 to 58 μg/Sm3 at the BF inlet and 3 μg/Sm3 to 42 μg/Sm3 in stack emissions. Removal efficiency ranged from 77% to 28%. Fig. 2 shows the variations in Hg speciation before the BF and stack in the tested CKs. Among the heavy metals measured in this study, transformation of Hg within APCDs was more complex due to its high volatility, interaction with flue gas components, and presence of fly ash. Due to the typical features of the kiln temperature gradient, there is an inherent recycling of Hg within the system, and thus it may take a long time for it to reach a steady state.27)
Mercury speciation and removal efficiency was similar between CK #1 and CK #2 but was somewhat different in CK #3. Mercury speciation before BF was predominantly in elemental form (ranging 75.1%, to 98.8%). By passing through the BF portion, the proportion of oxidized Hg in all cases increased (5.6% to 80.4%). Earlier studies have also shown the oxidation tendency of elemental Hg across BF.28) The oxidation and speciation variations between the facilities may be related to the chlorine content in fuel, acidic gas composition in flue gas, fly ash, and residence time of flue gas within the system. The variation of Hg speciation and
emission concentration may be related to chlorine concentration, flue gas components, and efficiency of APCDs.29-32)
There is no well-established technology that can control Hg emissions across the entire utility industry. Various methods are being studied, and emerging control technologies are relatively new and have not yet been tested on a commercial scale. Sorbent injection, elemental Hg oxidation, and multipollutant control technologies have been extensively studied so far. Air pollution control devices installed for removing nitrogen oxides, sulfur oxides, and particulate matter (PM) can achieve co-benefits of Hg capture. Higher co-benefits of Hg control in CKs can be achieved in facilities equipped with PM control devices and wet APCDs, since oxidized Hg is soluble in water and can be removed in a wet scrubber solution.
One of the major concerns with CK operation is to manage CKD-alkaline waste removed from APCDs with high concentrations of heavy metals. The higher alkali content in CK and in the scrubber favors retention of metals and its compounds in clinker and CKD.21) The U.S. EPA classifies CKD as a “special waste,” and it has been temporarily exempted from federal hazardous waste regulations under Subtitle C of the Resource Conservation and Recovery Act.33) Depending upon the pollutant levels, some CKD can be reused directly, and some requires treatment before reuse. The U.S. EPA is in the process of developing standards for the management of CKD and has published a set of proposed subtitle D (i.e., non-hazardous, solid waste) regulations to govern CKD management. To control the release of CKD into the air, the U.S. EPA has proposed a performance standard to prevent release from landfills, handling conveyances, or storage areas. As an alternative to the performance-based standard, technology-based standards are proposed.
Sixty percents of Hg and nearly all of Be, Cd, and As entering BF are distributed in CKD (Fig. 3). The cycling of volatile metals as a result of CKD recycling (metals accumulate in CKD through repeated pass through kiln) must be handled properly to meet all the environmental management standards.
Here, it is worthwhile to note that when emission limits become stringent, cement plants are forced to install more efficient APCDs, and a larger proportion of metals are thereby captured in CKD along with particulate removal. Recycling of CKD, a common practice during cement production, increases the metal content in cement, the final product. Metals in concrete are low-leachable
and are long-term stable.34,35) However, allergic contact dermatitis, a potentially serious condition, can result to permanent disability when wet cement containing Cr (VI) comes in contact with skin. Thus, proper recycling and management of CKD is essential. The European Union directive 2003/53/EC prohibits the use or sale of cement or cement preparation with higher soluble Cr (VI).36)
3.5. Volatile Organic Compounds Emission
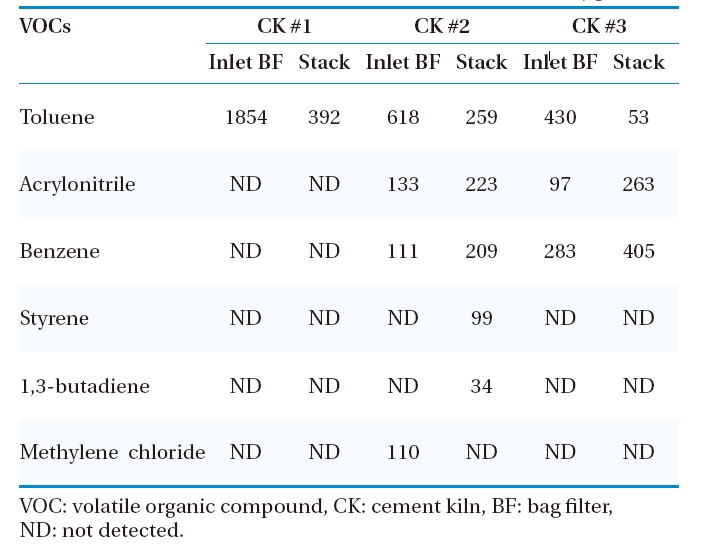
The presence of VOCs in the emission of the combustion unit indicates an inefficient process. Benzene, acrylonitrile, and toluene were the major VOCs emitted from CKs tested (Table 5). Here, it is notable that few VOCs increased in concentration in the stack emission, because the samplings at these points were taken at different time. The other VOCs tested?vinyl chloride, chloroethane, cis-1,2-dichloroethylene, chloroform, carbon tetrachloride, trichloroethylene, tetrachloroethylene,?chlorobenzene, ethylbenzene,?m,p-xylenes, and o-xylene?were not detected before BF and stack emissions. Volatile organic compounds such as benzene, toluene, chloroform, trihalomethane and di-, tri-, and hexachlorinated benzene are usually present in the combustion flue gas. Trihalomethane, for instance, comes from process water rather than the combustion process, and carbon tetrachloride and chlorobenzene are often used for spiking feed material during performance testing in order to increase destruction and removal efficiency, so they also may appear as a product of incomplete combustion.37) The characteristic residence time of combustion gases in CK is more than five seconds at temperatures higher than 1,000C, whereas it is only two seconds in the typical incineration process. This favors a highly efficient environment for destruction of the organic components of fuel and waste used. Other VOC compounds, of higher concentrations, from other incineration facilities such as municipal waste incinerator (MWI) have been measured in Korea.38)
[Table 5.] VOC emissions before BF and stack of cement kilns (μg/Sm3)
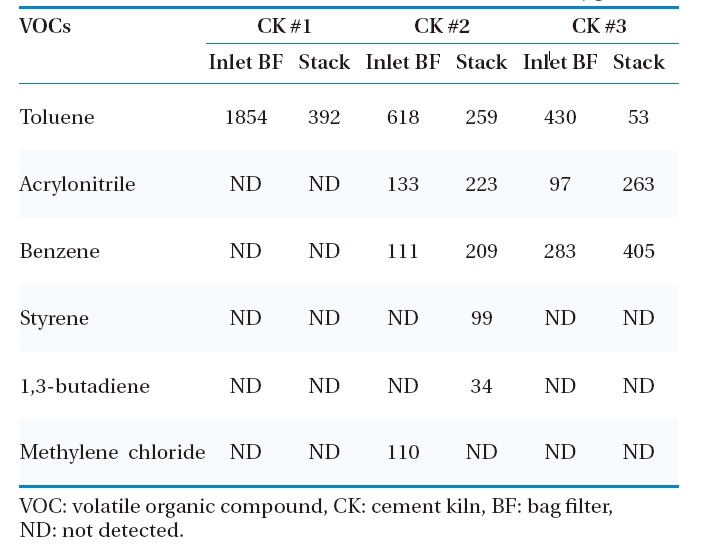
VOC emissions before BF and stack of cement kilns (μg/Sm3)
Here, it is notable that all the VOCs species (toluene, acrylonitrile, benzene, styrene, 1,3-butadiene, methylene chloride) present in the flue gas are among the 187 listed HAPs by U.S. EPA. Thus, in spite of their low concentrations, proper consideration is required to minimize its emission.
The major heavy metals emitted from CKs co-burning waste were Hg, Zn, Ni, Cr, Pb, Cd, and As. Removal efficiency of heavy metals (except Hg) was above 98.5%; the higher removal efficiency was due to the use of high-efficiency BF in all the kilns tested. The average Hg removal efficiency of BF was above 60%. On average, 3.3% of the medium- and low-toxicity heavy metals (Pb, Ni, Cr) entering the BF were released into the atmosphere. 0.14% of the Cd and 0.01% of the As entering BF were released into the atmosphere, while 40% of the Hg-one of the highly toxic metals?was emitted in the atmosphere. Emission variations of metals among the CKs tested were related to raw materials, fuel, and waste-feed and operating conditions. The cycling of volatile metals as a result of CKD recycling must be handled properly to ensure that all of the environmental management standards are met. Volatile organic compounds emissions from CKs were smaller, but all the VOC species (toluene, acrylonitrile benzene, styrene, 1,3-butadiene, methylene chloride) detected in emission gas are HAPs. So, in spite of their low concentration, proper attention is required to minimize their emissions.