Since the discovery of carbon nanotubes (CNT), functional composites including CNT have attracted considerable attention in the research and industrial communities, due to their special characteristics, such as high chemical stability, extraordinary mechanical and unique electronic properties and adsorption1,2) ; furthermore, it can absorb organic substance strongly with particular π-electron structure.3)
On the other hand, TiO2 has been widely used in solving various environmental problems such as wastewater treatment, air clean, as a photocatalyst.4-6) In order to improve the photocatalytic efficiency at TiO2 surfaces, a great deal of effort has been devoted7-9) the activity of TiO2 as photocatalyst is well recognized to depend strongly upon the method of preparation.10) The sol-gel route is well established as an excellent method to prepare the TiO2-based materials, including modification by incorporating foreign ions,11) TiO2 film by a dip-coating process12) and titanium/carbon composites.13-16) Provided that CNTs is used as support material and all those advantages of both CNTs and TiO2 are utilized a powerful photocatalyst could be produced.
It is very important work that improving comprehensive photocatalytic performance of CNTs and TiO2 through combining they better. There are some endeavors to combine CNTs with TiO2 to form catalyst composites, Wang17) reported that Multiwalled carbon nanotubes (MWCNT) and nanocrystalline titanium composite catalysts were prepared by a modified acid-catalyzed sol-gel method. Hernadi18) reported that MWCNT-based titanium composite material has been prepared by an impregnation method, which provides a homogeneous inorganic cover layer on the surface of purified MWCNT. Rutile TiO2 has been immobilized on the sidewall of MWCNT by a simple one-step scheme, which produces three distinct morphologies of hybrid MWCNT at different reaction temperatures.19) Coating MWCNT surface with TiO2 has been performed by a sol-gel method using different alkoxides and by hydrothermal hydrolysis of TiOSO4, which can lead to different morphologies.20)
The common characteristics of the above CNT-TiO2 composites are the TiO2 particles linked or coated on CNTs. In this study, we focused on the characterization of the CNT/TiO2 com posites electrodes prepared through impregnation with titanium n-butoxide (TNB, Ti(OC4H7)) solution, which the CNT matrix were impregnated with different concentration TNB solution to form CNT/TiO2 composite electrodes. The developed electrodes were characterized by BET surface area, X-ray diffraction (XRD), scanning electron microscope (SEM), energy dispersive X-ray (EDX). The catalytic efficiency of the developed electrode was evaluated by the photoelectrodegradation of an azo compound, methylene blue (MB, C16H18N3S?Cl?3H2O).
CNTs were selected as the support material. The CNTs, supplied from carbon nano-material technology Co., Ltd, Korea. Korea (Multiwall nanotubes, diameter: ∼20 nm, length: ∼5 μm), were used without further purification. The TNB as a titanium source for the preparation of CNT/TiO2 composites were purchased from Acros Organics, New jersey, USA. The novolac typed polymer resin was supplied from Kangnam Chemical Co., Ltd, Korea. The MB was used as analytical grade which was purchased from Duksan Pure Chemical Co., Ltd, Korea. It was selected because it can be readily under anaerobic conditions to produce potentially more hazardous aromatic amines.
2.2. Preparation of CNT/TiO2 Electrodes
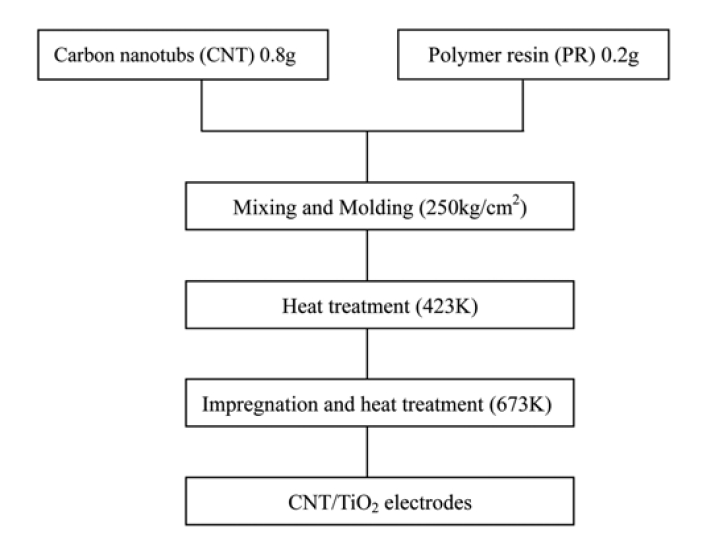
Several CNT/TiO2 electrodes were prepared. The preparation procedure for the electrodes is given in Fig. 1. The CNT/TiO2 electrodes were pressed at the pressure of 250 kg/cm2 in a mould, the dimension of CNT/TiO2 electrodes was 9.95 mm × 39.5 mm × 5.95 mm. The curing temperature of the CNT matrix was 423 K, and then the cured sample pyrolyzed at 673 K for 1h in order to completely cure the binder. The prepared CNT matrix were impregnated with different concentration TNB solution for 30 min., and then heat treated at 673 K for 1h to form CNT/ TiO2 composite electrodes. The preparation conditions and the sample code used in this study were listed in Table 1.
2.3. Characteristics and Investigations of the Samples
The Brunauer-Emett-Teller (BET) surface area of the CNT/ TiO2 composites was evaluated from N2 adsorption isotherm at 77 K using a BEL Sorp Analyzer (BEL, Japan). XRD was used for crystal phase identification and estimation of the anastaseto- rutile ratio. XRD patterns were obtained at room temperature with a diffractometer Shimata XD-D1 (Japan) using Cu Kα radiation. SEM was used to observe the surface state and porous
structure of the CNT/TiO2 composites were carried out using a JSM-5200 JOEL electron microscope (Japan). EDX was used to measure the elemental analysis of the CNT/TiO2 composites. UV-VIS spectra for the MB solution obtained from degradation by CNT/TiO2 composites dispersion under different conditions were recorded using a Genspec III (Hitachi, Japan) spectrometer.
2.4. Photoelectrocatalytic (PEC) Decompositions
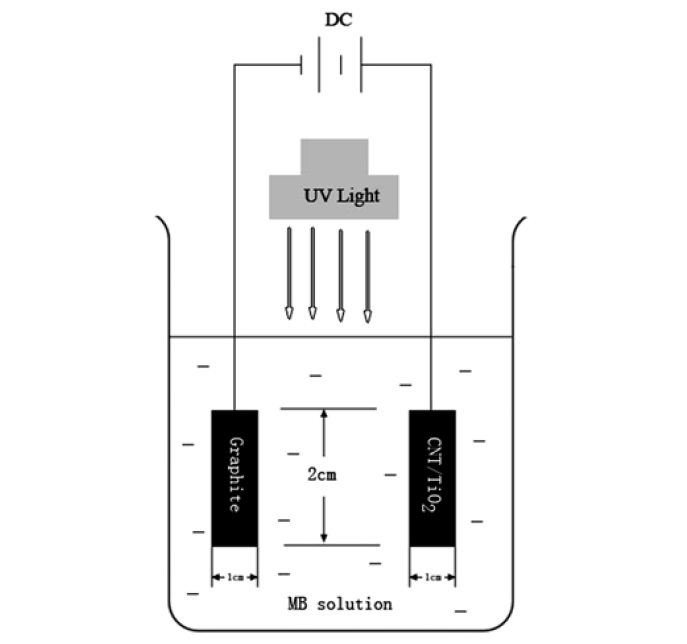
The PEC decomposition was performed by using CNT/TiO2 electrode as the test electrode and an aqueous solution of MB in a 100 mL glass container and then irradiating the system with 20 W UV light at 365 nm, which was used at the distance of 100 mm from the solution in darkness box. The counter electrode was artificial graphite (TCK, Korea), which dimension was 9.95 mm × 39.5 mm × 5.95 mm. The same CNT/TiO2 electrode was placed in 50 mL of 1.0 × 10-5 mol/L MB solution. The photoelectrocatalytic degradation of MB was performed with a potential voltage of 3.0 v and UV light (Fig. 2). The photoelectrocatalytic activities of the CNT/TiO2 electrodes were investigated using the photoelectrodegradation rate of MB, which was measured for 10 min, 20 min, 30 min, 40 min, 50 min and 60 min. The blue color of the solution faded gradually with time due to the adsorption and decomposition of MB. And then the concentration of MB in the solution was determined as a function of irradiation time from the absorbance change at a wavelength of 660 nm.
3.1. Structure and Morphology of CNT/TiO2 Composites
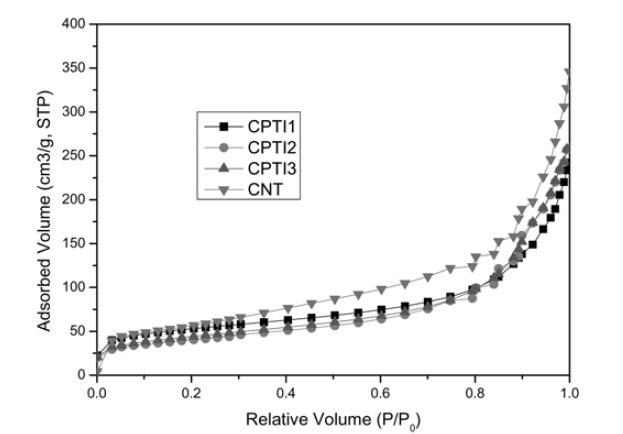
The N2 adsorption isotherms for CNT/TiO2 composites are
[Table 1] Nomenclatures of CNT/TiO2 electrodes

Nomenclatures of CNT/TiO2 electrodes
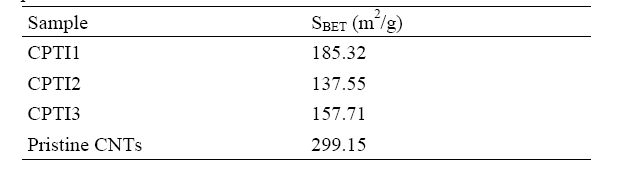
shown in Fig. 3. Fig. 3 shows an idealized form of the adsorption isotherm for physisorption on CNT/TiO2 composites. At low pressures the surface is only partially occupied by the gas, until at higher pressures the monolayer is filled and the isotherm reaches a plateau. Judged from above (approved by IUPAC) this is a Type II adsorption isotherm. All of the isotherms can be ascribed to type II, which suggests a mixed micro-and mesoporous texture. The values of BET surface area of CNT/TiO2 composites were shown in Table 2. As the results of Table 2, the BET surface areas of pristine CNTs is 299.15 m2/g, while the BET surface areas of CPTI1, CPTI2 and CPTI3 are 185.32 m2/g, 137.55 m2/g and 157.71 m2/g, respectively, it demonstrated that all of the BET surface areas for these composites were a considerable decrease than that of pristine CNT, which suggesting that some porosity was developed during the heat treatment. On the one hand, this could be attributed to the partly blocking of micropores by the formation of TiO2 on the CNTs surface with the heat treatment. On the other hand, the BET surface area decreased due to the curing of polymer resin with heat treatment, which blocked the micropores and formed some new mesopores. It was possible that the polymer resin had coated some CNT particles to form some larger composite particles during the hardening process. The same phenomena can be well confirmed by the SEM morphology of CNT/TiO2 composites.
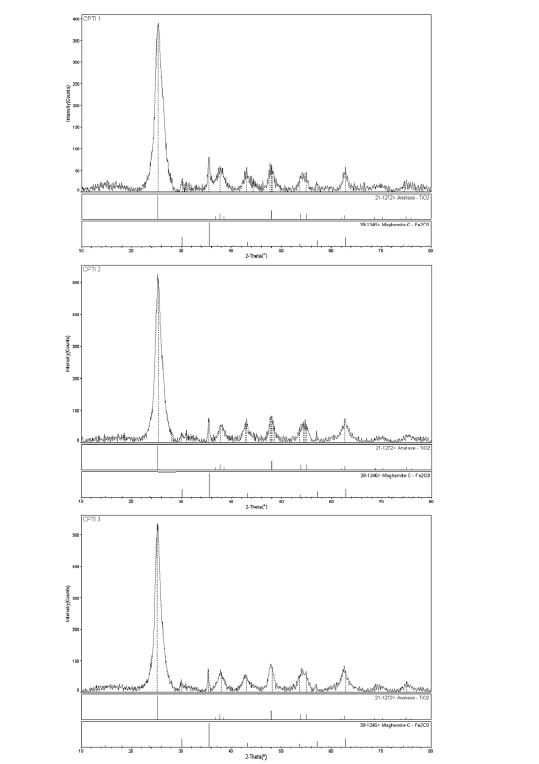
The XRD results for the catalyst samples are shown in Fig. 4. The structure for the CNT/TiO2 composites shows a typical single and clear anatase crystal structure. It is well known that the crystal structure of the titanium dioxide is mainly determined by the heat treated temperature. After the heat treatment at 673 K for 1 h, the main crystalline phase is not transformed to the rutile structure. The major peaks at 25.3, 37.8, 48.0, 53.8 and 62.5 are the diffractions of (101), (004), (200), and (105) and (204) planes of anatase, indicating the developed CNT/TiO2
[Table 2] Textural properties of pristine materials and CNT/TiO2
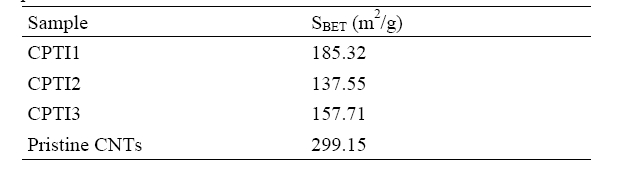
Textural properties of pristine materials and CNT/TiO2
composites existed in anatase crystal phase. As we known, the anatase phase formed below 773 K starts to transform to rutiletype structure above 873 K and changed into single phase of rutile at 973 K-1173 K.21) The result is in accordance with,22) so the results of XRD in this paper are reasonably.
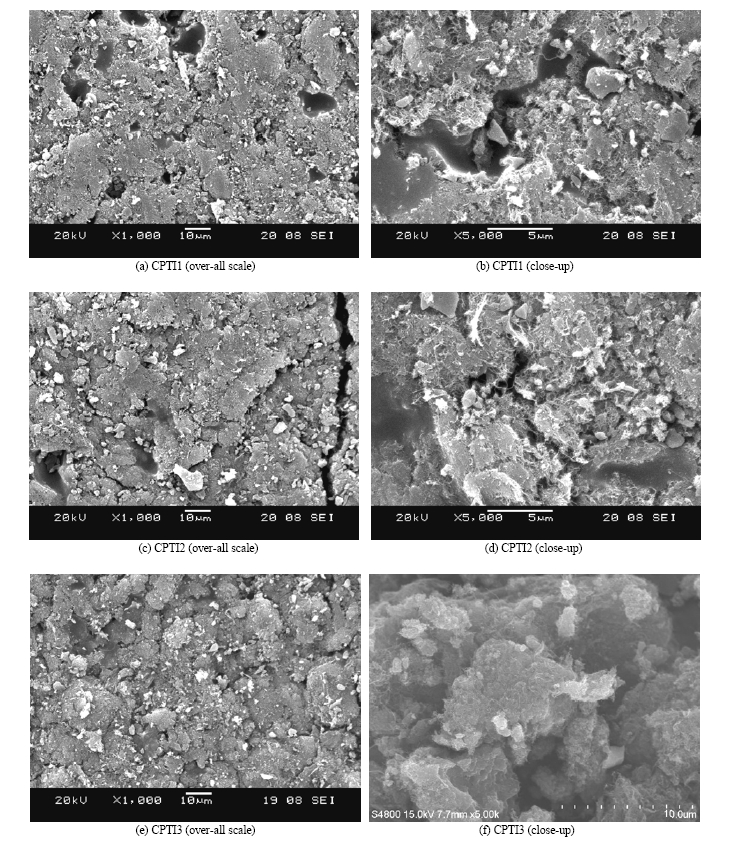
The micro-surface structures and morphology of CNT/TiO2 composites prepared with different concentration TNB solution were characterized by SEM and FE-SEM. Fig. 5 shows the changes in the morphology of CNT/TiO2 composites. According to the Fig. 5, in case of CPTI1, the particles of TiO2 were well distributed among the CNTs network, and some TiO2 particles aggregated to be small clusters, this result is as well confirmed by FE-SEM inspection of CNT/TiO2 composites. According to the Fig. 5(f), the image presents a close-up scale view of TiO2 introduced from composites with external diameters ranging from 1.5 to 3.0 μm. It was considered that a good dispersion of small particles could provide more reactive sites for the reactants than aggregated particles. But it was also observed that the dispersion of the TiO2 particles for all CNT/TiO2 composites prepared with different TNB concentration was not evidently changed among the CNTs networks. Therefore, the higher photocatalytic activity of the CNT/TiO2 composites prepared might be attributed to chemical degradation. At the same time, the conductive of CNTs network can facilitate the electron transfer between the adsorbed MB molecules and the catalyst substrate23-26); it was beneficial for the PEC reaction because the PEC reaction is carried out on the surface of the CNT/TiO2 composites catalysts and the CNTs network. So the CNT/TiO2 composites
would show an excellent PEC activity.
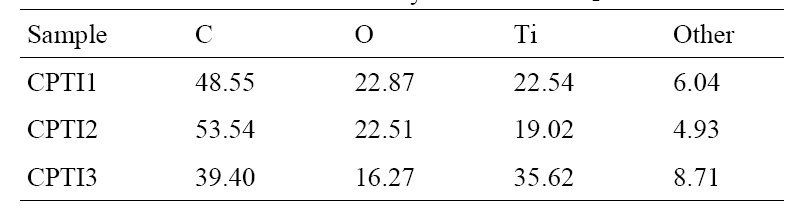
The results of EDX elemental microanalysis of CNT/TiO2 composites were listed in Table 3. From the EDX data, the main elements such as C, O and Ti were existed and other impure elements were also existed. The Ti content percents of CPTI1, CPTI2 and CPTI3 are 22.54%, 19.02% and 35.62%, respecti-
[Table 3] EDX elemental microanalysis of CNT/TiO2 electrodes
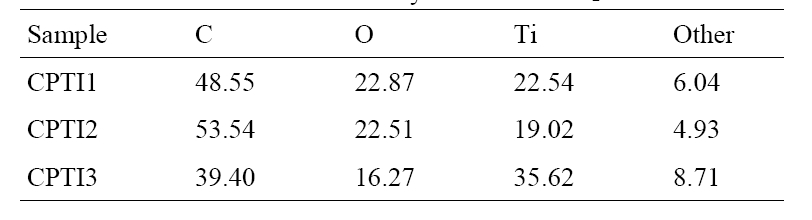
EDX elemental microanalysis of CNT/TiO2 electrodes
vely. It was not observed that the Ti element contents in the composites increased with an increase of TNB concentration. However, The Ti content of CPTI2 was some lower than expected. It was proposed that the Ti content introduced into the composites has been affected by the pore structure of the prepared CNT matrix. The same phenomena can be observed in the structure of CNT/TiO2 composites.
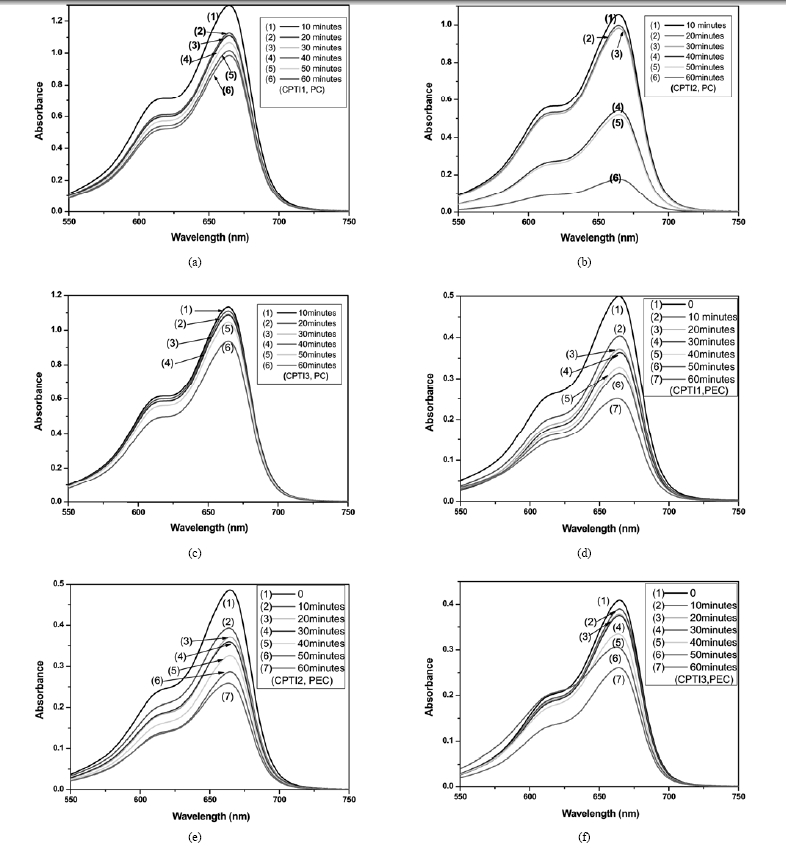
The UV/VIS absorbance spectra of MB concentration of 1×10-5 mol/L with the different CNT/ TiO2 electrode under various time conditions are shown in Fig. 6. As can be seen from the spectra Fig. 6, The absorbance maxima for all samples decrease with an increase of UV irradiation time. This can be indicated that the
color of MB solution is removed increasingly, so the concentration of MB solution is also decreased increasingly. From the Fig. 6(a), (b), (c), we can also observe that the absorbance maxima of the sample CPTI2 is much more decreased than that of the sample CPTI1 and CPTI3 in the same irradiation time. According to the former studies,14-16,27) we can consider that the CPTI2 will have better PC degradation of MB solution than that of the CPTI1 and CPTI3. However, the Ti content of CPTI2 is lowest among the three samples. Comparing with the Fig. 6(d), (e), (f), it can be seen that the absorbance maxima for all samples decrease by photocatalytic effect of CNT/ TiO2 electrode are more high than that of PEC effect. It can be evidently indicated that two types of degradation of MB were electro-assistant activity of CNTs and PC performance of TiO2. So the efficiency of PEC oxidation for MB is higher than that of PC oxidation.
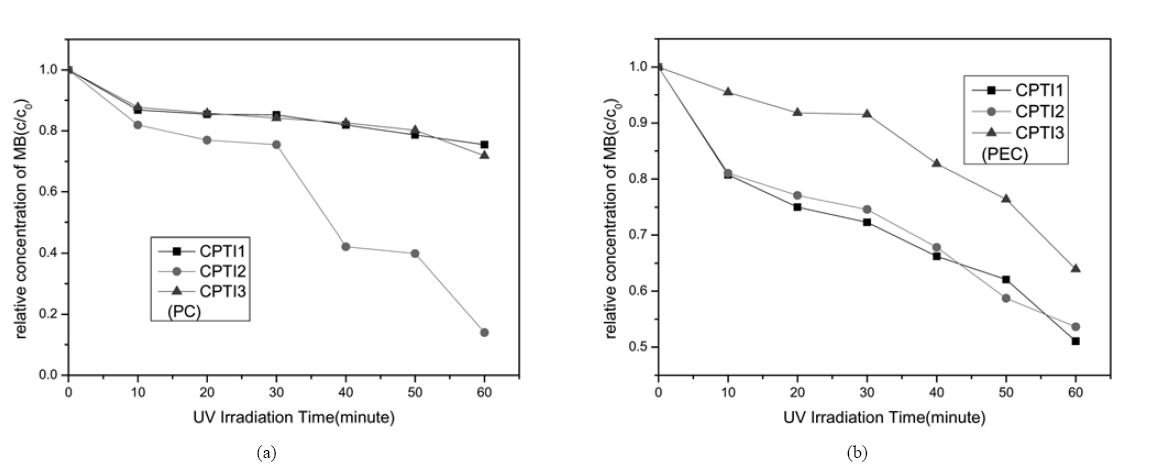
Fig. 7 shows the changes in relative concentration (c/c0) of the CNTs/TiO2 composites in MB concentration of 1×10-5 mol/L under UV irradiation with or without electron current in the aqueous solution. From the present results in Fig. 7(a), it can be seen that the PC process of MB with fast degradation efficiency of CPTI2 was observed. It is considered that decrease of MB concentration in the aqueous solution can be occurred in two physical phenomena such as adsorption by CNTs and PC decomposition by TiO2, and that the adsorption by CNTs was mainly here. As the result of EDX, the CPTI2 has the most content of carbon and the lowest content of Ti. See from the Fig. 7(b), the PEC oxidation of CPTI1 and CPTI2 are higher than that of CPTI3, it was possible that the PEC oxidation increased with an increase of CNTs composition, the carbon content of CPTI3 is the lowest among the three samples, so the CNTs electro-assisted effect of CPTI3 is lesser than that of CPTI1 and CPTI2. According to the former studies,27,28) it was possible that catalytic decomposition of MB solution could be attributed to combination effects between TiO2 photocatalytic and CNTs electro- assisted.
3.3. Degradation Mechanism Analysis
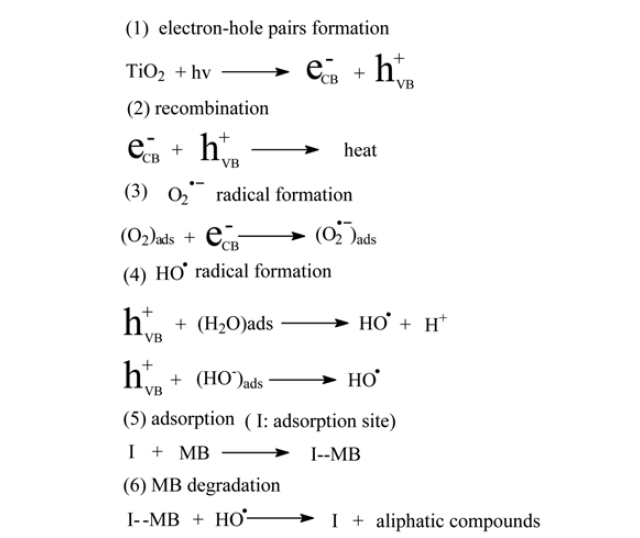
The mechanism of the PC oxidation using CNT/TiO2 composites electrodes can be summarized in Fig. 8.29-31) Under UV illumination, the excited high-energy states of electron-hole pairs are formed in TiO2 particles (in reaction (1)). Part of these photogenerated carriers recombine in the bulk of the semiconductor (in reaction (2)), while the rest migrate to the surface of particles, where the holes act as powerful oxidants and the electrons as powerful reductants and initiate a wide range of chemical redox reactions, which can lead to complete mineralization of the dyes. Since OH- and H2O are the most abundant adsorbates, it is likely that holes will react with these species, and then form the HO? radicals (in reaction (4)), which these extremely powerful oxidizing agents can nonselectively attack the adsorbed organic molecules or those close to the catalyst surface. the PC reaction occurs when the HO? radical meeting MB molecule adsorbed on the surface of particles (in reaction (5),(6)), the HO? radicals can attack some functional groups in MB, causing MB concentration decrease, then the MB molecule decomposed from aromatic compounds into some aliphatic compounds, thus, resulting to their photodecompostion. The decrease of MB through reaction (6) is important to improve the photocatalytic effect of CNT/TiO2 electrodes, it can consume HO? from TiO2 promoting of the HO? production(reaction (4)), on the same time, reduce the recombination of hole/electron pairs(reaction (2)), which causing the catalytic capability of the composite was greatly improved. On the other hand The O2 adsorbed on the surface of particles will react with the photoinduced electrons reducing the competing reaction (2) rate, and then more holes will attend reaction (6). The above scheme is reasonably depict the photodegradation process of MB. In our case, the applied potential was in PEC reaction, the recombination of photogenerated hole/ electron pairs was suppressed by the externally applied electric field, and thus the life of the holes and electrons got longer,32) so the conductivity of CNTs network facilitates the electron
transfer between the adsorbed MB molecules and the catalyst substrate.
In this study, we present the fabrication and characterization of CNT/TiO2 composites electrodes. The BET surface areas for CNT/TiO2 composites decreased with an increase of TiO2 components. XRD data revealed that all samples had an anatase typed titanium dioxide structure. The SEM and FE-SEM microphotographs of CNT / TiO2 composites showed that the particles of TiO2 were well distributed among the CNTs networks, and some TiO2 particles aggregated to be small clusters. From the EDX data, the main elements such as C, O and Ti were existed. The photodegradation of MB using the prepared electrode under different experimental conditions was investigated in terms of UV absorbance. The results demonstrated that the PEC oxidation of MB concentration in the aqueous solution can be contributed to the combination effects of PC decomposition by TiO2 and CNTs network electro-assisted, Through the comparison between PC oxidation and PEC oxidation, it was found that the PEC oxidation was a more convenient and effective way to mineralize the organic matters.