Pharmacopuncture is an extract that is produced using different techniques, depending on the characteristics of the herbs, their shapes, and their usage [1]. Meridian field pharmacopuncture (MFP) is a famous pharmacopuncture using the six meridian fields (wind, heat, fire, dry, cold, damp) that reflects one’s health. MFP is divided into two different types, Aromatic and oil based pharmacopunctures. Carthami flos (CF) pharmacopuncture is a representative oil based pharmacopuncture.
Many studies have already verified the efficacy of CF. Lee
Although CF treatment has many benefits, adverse effects like pain, erythema, and swelling also occur with the use of CF [6, 7]. These adverse effects usually appear after repeated injections or over dosed injections, but are limited to the local injection points. Water-soluble carthami flos (WCF) is a new mixture of CF, water for injection (WFI), and a natural emulsifier. It was developed to increase absorptivity and decrease the adverse effects of CF [8]. Currently, several reports on the safety of WCF have been published Lee
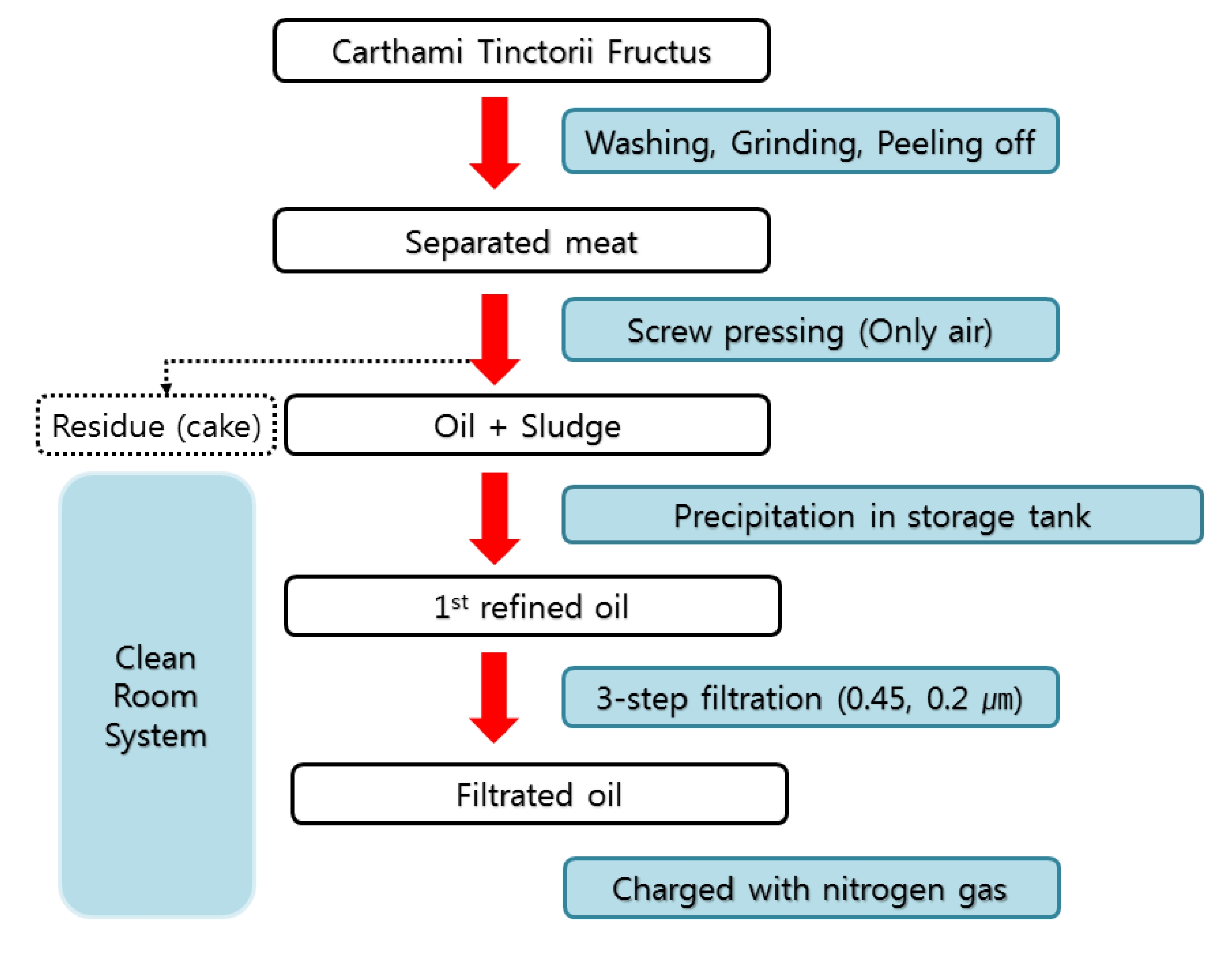
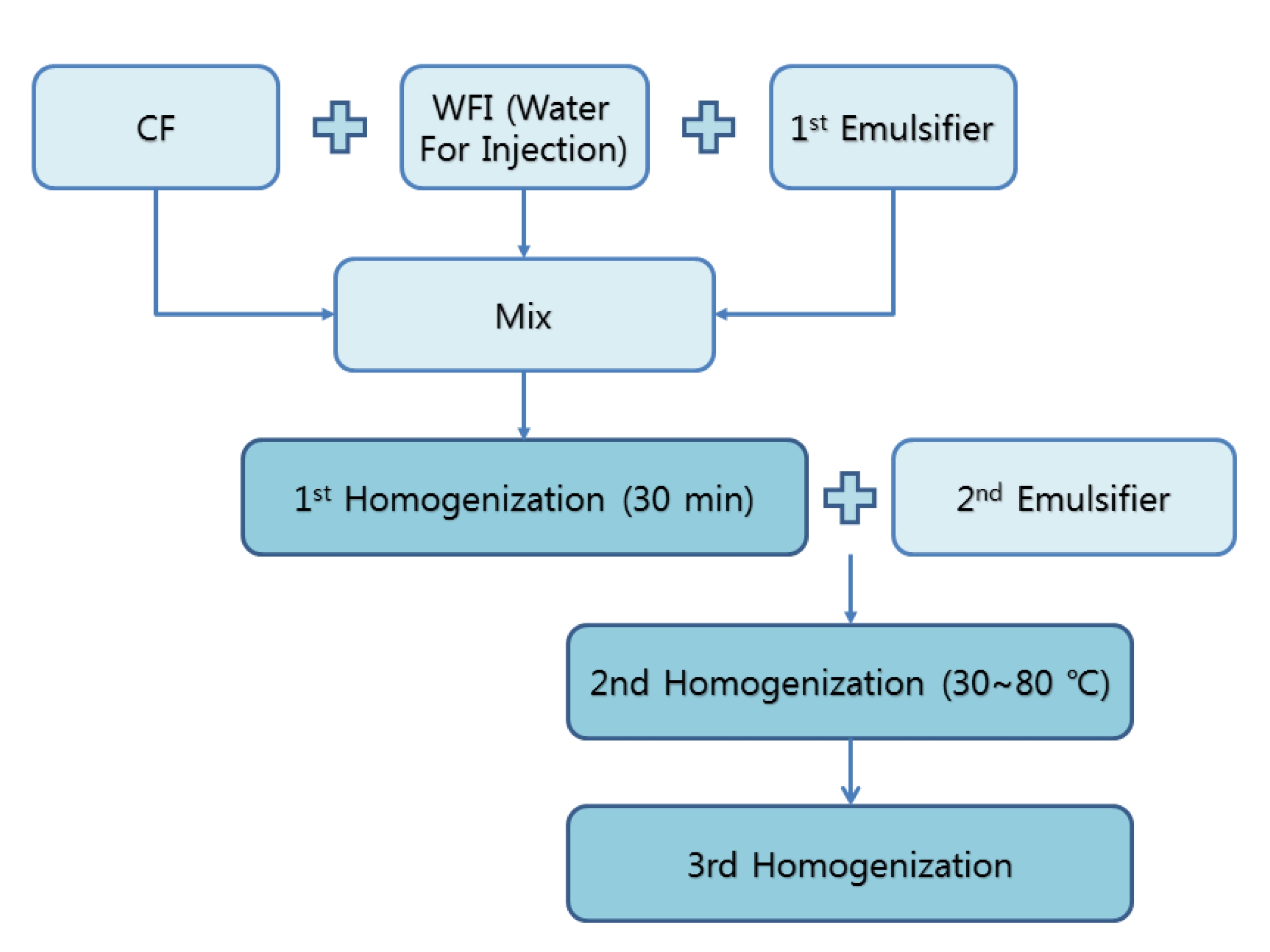
The WCF solution was provided by the Korean Pharmacopuncture Institute (KPI). The manufacturing steps of CF are as follows [10] (Fig. 1): The
SD rats were chosen for this study because that they have been widely used in drug safety test, so many comparable databases already exist. Forty-six male and 46 female, 5-week old SD rats were purchased from Orientbio Inc. Visual inspections and measurements of body weight (electronic scale, CP3202S, Sartorius, Germany) were done at the time of purchase. The body weights of the male and the female rats were in the ranges of 123.6 – 143.8 g and 103.4 – 127.2 g, respectively.
The feeding environment was as follows: The temperature of the laboratory was 22.2 – 23.8℃, the relative humidity was 44.5% – 61.3%, the air was changed at a rate of 10 – 15 ventilations/hour, the cycle of light and shade was 12 hours/day (7 am to 7 pm), and the illuminance was 150 – 300 Lux. The rats were fed freely (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and supplied enough clean water (tap water filtered by using a UV sterilizer and irradiated with UV rays).
The clinical signs of the rats were observed during six or seven days of acclimation. At the end of the acclimation period, the body weights of the male rats were in the range 186.3 – 207.7 g, and those of the female rats were in the range 140.0 – 179.9 g.
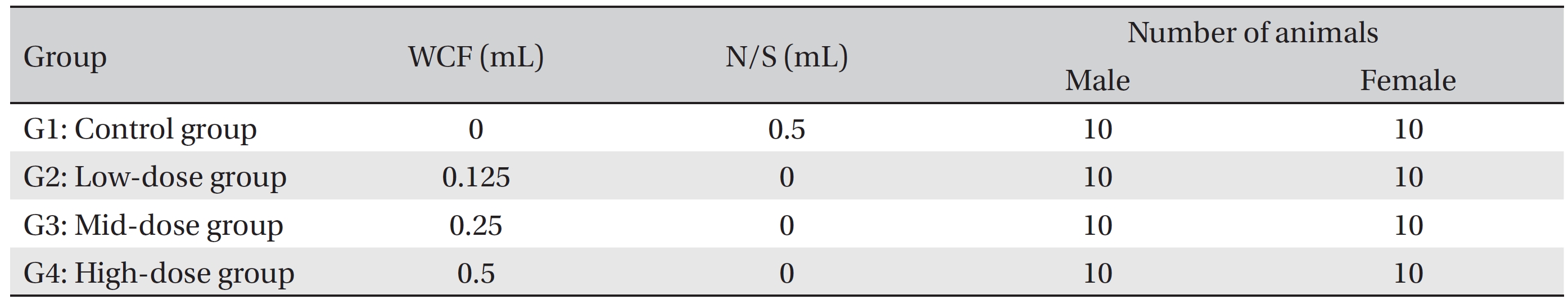
Group separation was done on the last day of acclimation. We selected 6-week old SD rats for each sex if they were close to the average body weight. The 80 animals were randomly divided into 4 different groups with 10 individuals of each sex per group: Group 1 (G1, control group) was administered a normal saline solution (Choongwae Pharma Corp., Korea) while Group 2 (G2, low-dose group), Group 3 (G3, mid-dose group), and Group 4 (G4, high-dose group) were administered 0.125, 0.25 and 0.5 mL of WCF/ animal/day, respectively. Previously, a report stated that no deaths and no clinical signs had been observed for a 1 mL/animal/day, single, intramuscular dose of WCF in rats (Biotoxtech Study No.: B12873). Accordingly, a dose of 0.5 mL/animal/day was selected for the high-dose group and same amount of normal saline was selected for the control group. The remaining animals were excluded (Table 1).
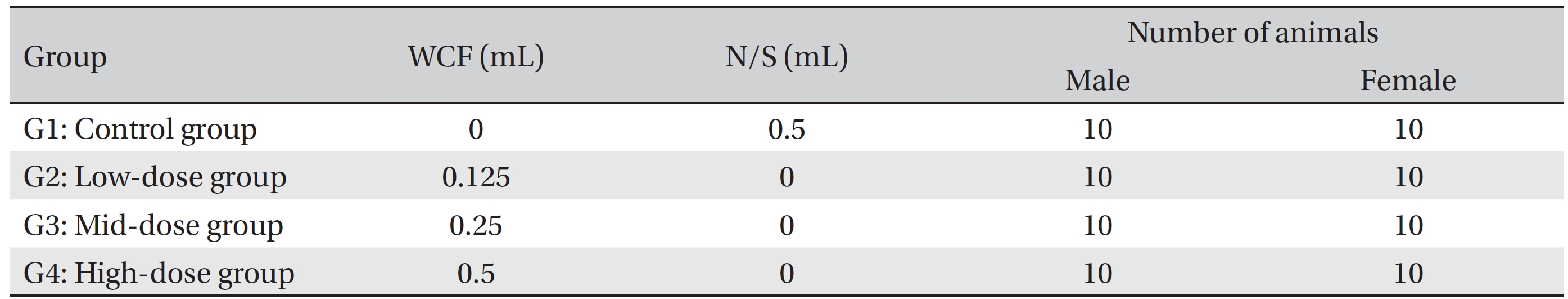
Groupings of rats
The route of administration of this study was intra-muscular (IM) injection because the expected route of WCF in clinical application is to muscle. Injections were performed using disposable syringes (0.3 or 1 mL, 30 G). In each group, each solution was injected into the femoral muscle of the rats. Injections were done once a day, alternating from side to side, for 4 weeks. This study was conducted under the regulation of Good Laboratory Practice (GLP) of the Korea Food and Drug Administration (KFDA, KFDA Notification No. 2013-40) and the approval of the Institutional Animal Care and Use Committee (IACUC, Approval No. 130412).
During the study, we observed clinical signs of all rats once a day and checked the number of deaths and presence of critical conditions twice a day. The body weight of each rat was measured by using an electronic scale (CP3202S, Sartorius, Germany) on the first day (before injection), weekly for 4 weeks, and on the day of necropsy. However, the last measurement was excluded from the statistical analysis because all rats had been fasting for more than 18 hours before necropsy. The mean food consumption was measured by averaging the consumptions on 21 days and 6 days of the last week. To compare food consumption data during injection with that during a normal state, data before injection was measured from the day of grouping to the first day of injection.
Opthalmological examinations of five rats in each group were carried out during the last week. Pupillary light reflex and observation of the anterior segment of the eyeball were done before applying a mydriatic drug. After the use of a mydriatic drug (Lot No.: 12K21B, isopto atropine eye drops 1%, Alcon, Korea), the anterior segment, lens, vitreous body and fundus were examined by using an opthalmoscope (ALL PUPIL Ⅱ, Keeler, U.K.).
At the fourth week, urinalyses were conducted by using fresh or stored urine to assess the amount, specific gravity, pH, protein, glucose, ketone body, bilirubin, and occult blood in the urine. All rats were fasting for more than 18 hours before necropsy. Then, blood was drawn from the abdominal aorta on the day of necropsy, after having applied anesthesia with isoflurane. A 1-mL blood sample was put into an ethylene diaminetetra acetic acid (EDTA) tube and was analyzed by using a blood corpuscle analyzer (Adiva 2120i, Wiemens, Germany). Erythrocyte count (RBC), hemoglobin (HBG), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT), leucocyte count (WBC), WBC differential count (neutrophils, lymphocytes, monocytes, eosinophils, basophils) and reticulocytes (Reti) were measured. For the blood coagulation test, about 2 mL of the drawn blood was put into a tube containing 3.2% sodium citrate and was centrifuged for 10 minutes at 3,000 rpm before collecting the blood plasma. Measurements were done using a coagulation time analyzer (Coapresta 2000, Sekisui, Japan). The prothrombin time (PT) and the activated partial thromboplastin time (APTT) were measured.
The blood sample remaining after the hematological test was used in the clinical chemistry analyses. The blood was centrifuged for 10 minutes at 3,000 rpm, and the collected serum was analyzed by using a clinical chemistry analyzer (7180, Hitachi, Japan) and an electrolyte analyzer (ILyte, Instrumentation Laboratory, U.S.A.). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine (Crea), total bilirubin (T-Bili), total protein (TP), albumin (Alb), albumin/ globulin ratio (A/G ratio), total cholesterol (T-chol), triglycerides (TG), phosphorus (P), glucose (Glu), calcium (Ca), chloride (Cl), sodium (Na) and potassium (K) were measured.
All animals underwent necropsy on day 29. A visual inspection of the body organs and tissues was performed. The following organs were obtained from the animals and their absolute and relative weights were measured: brain, pituitary, heart, lung, liver, spleen, kidney, adrenal gland, testis, prostate, ovary, and uterus. The weights of bilateral organs, such as the kidneys, adrenal glands, testes, and ovaries were measured by the using the sum of the weights of both sides of the organ. The following organs and tissues were extracted and fixed in 10% neutral buffered formalin, and histopathological observations were carried out: brain, pituitary, thyroid and parathyroid, thymus, and lung, including bronchi, trachea, heart, liver, spleen, kidney, adrenal, salivary gland (submandibular, sublingual and parotid gland), esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, pancreas, testis, epididymis, prostate, seminal vesicle, ovary, uterus, vagina, urinary bladder, submandibular lymph node, mesenteric gland (inguinal), skin (inguinal), sternum including bone marrow, femur including bone marrow, tongue, spinal cord (thoracic), and other abnormal organs. The tissues were checked by using visual inspection. The injection site was also tested. Eyes and testes were preserved in Davidson’s fixative.
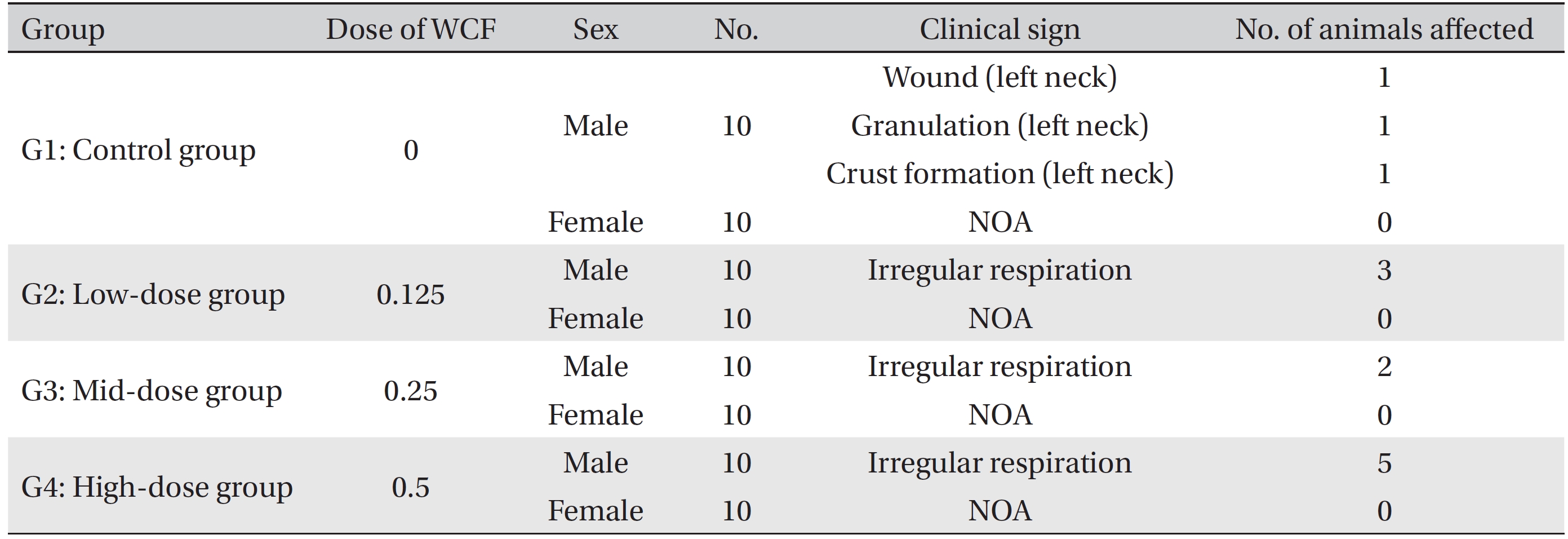
[Table. 2] Summary of clinical signs
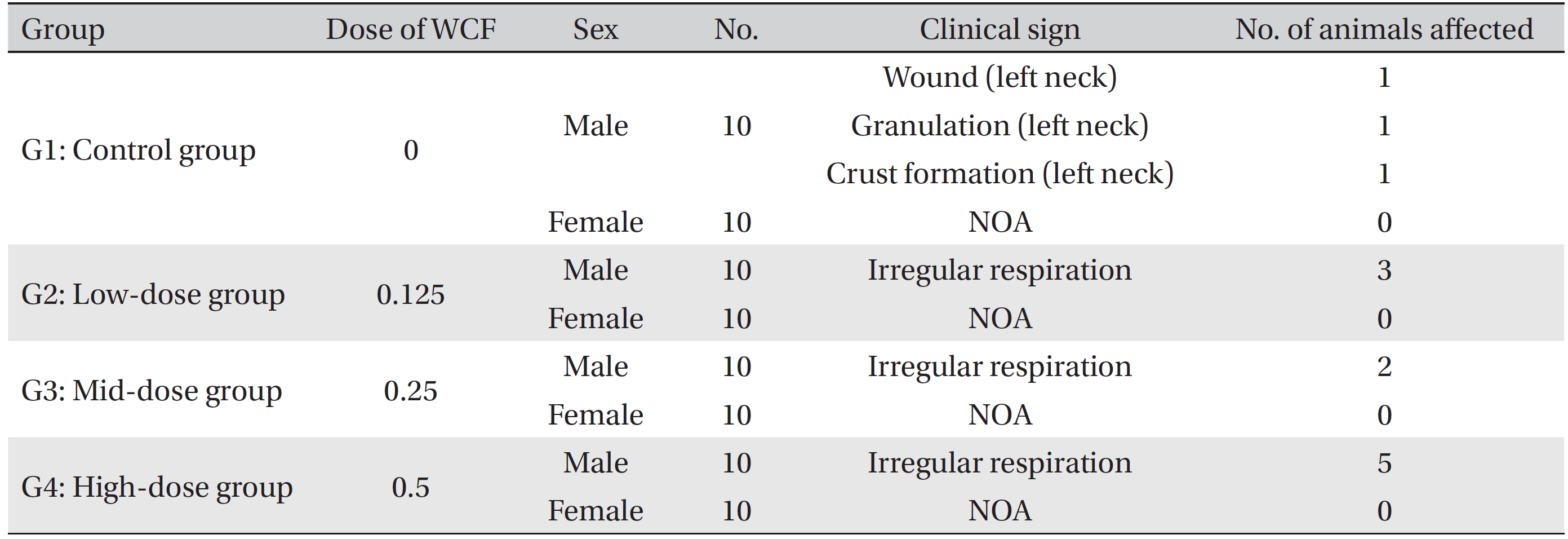
Summary of clinical signs
All data obtained from the rats were tested using statistical analysis system (SAS, version 9.3, SAS Institute Inc., U.S.A.). The Bartlett test was performed to check the homogeneity of the variance (significance level: 0.05). If the variance was homogeneous, the data were subjected to the one way analysis of variance (ANOVA) to yield significance (significance level: 0.05), and multiple testing of Dunnett’s
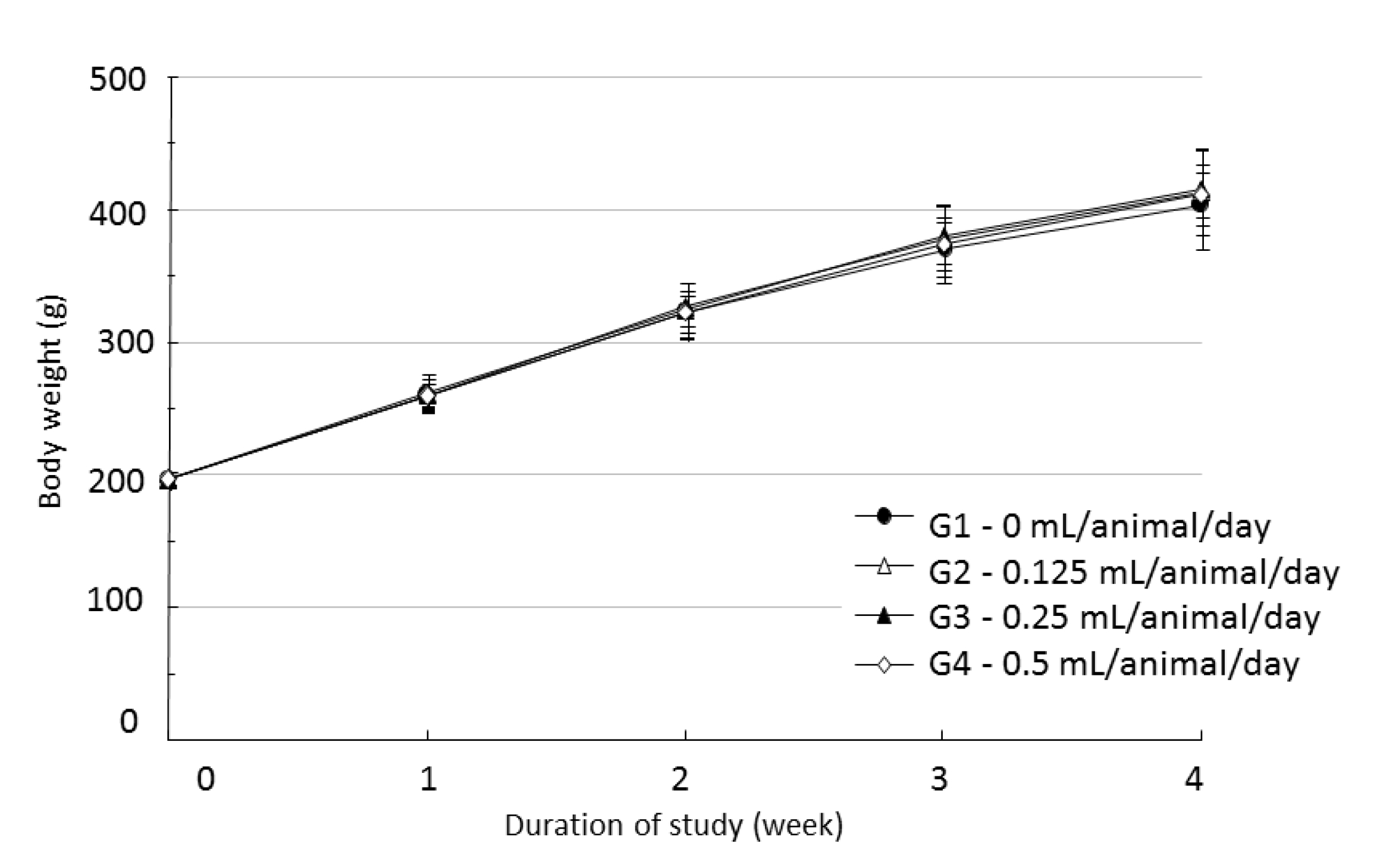
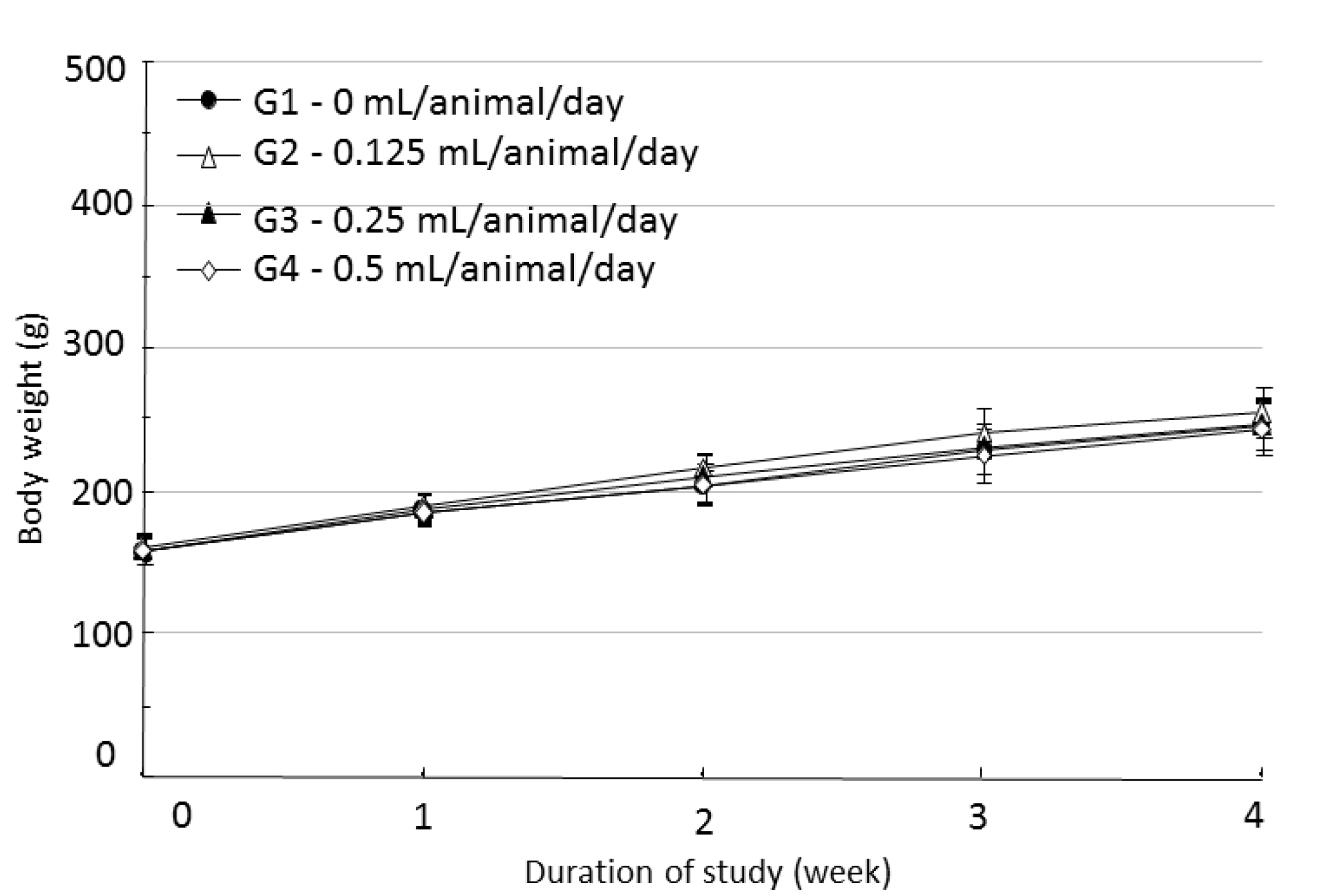
During the four weeks of the experiment, no deaths were observed. As a result, calculating the lethal dose for 50 percent kill (LD50) of WCF was impossible. However, we were able to verify that a repeated intramuscular dose of WCF at 0.5 mL/animal/day caused no deaths. No significant clinical signs were caused by injections into the female rats of the experimental groups, but three, two, and five male rats from groups G2, G3, G4, respectively, were found to have irregular respiration after WCF injection from day 1 to day 10. However, they soon recovered and no significant changes, such as weight loss compared to the control group were observed. Thus, the toxicity of WCF was not considered to be a cause of the irregular respiration. In addition, wounds, granulations and crust formations were observed on a male rat of the control group from day 13 to day 28 (Table 2). No significant changes in mean body weight (Figs. 3,4) and the mean food consumption between the experimental groups and the control group were noted.
In the opthalmological tests, no abnormalities were found. In the urinalyses, several changes were found in protein, glucose, ketone body, bilirubin etc., but they were found in the control group or were distributed randomly. Therefore, we concluded that the urinalyses revealed no significant toxicological results.
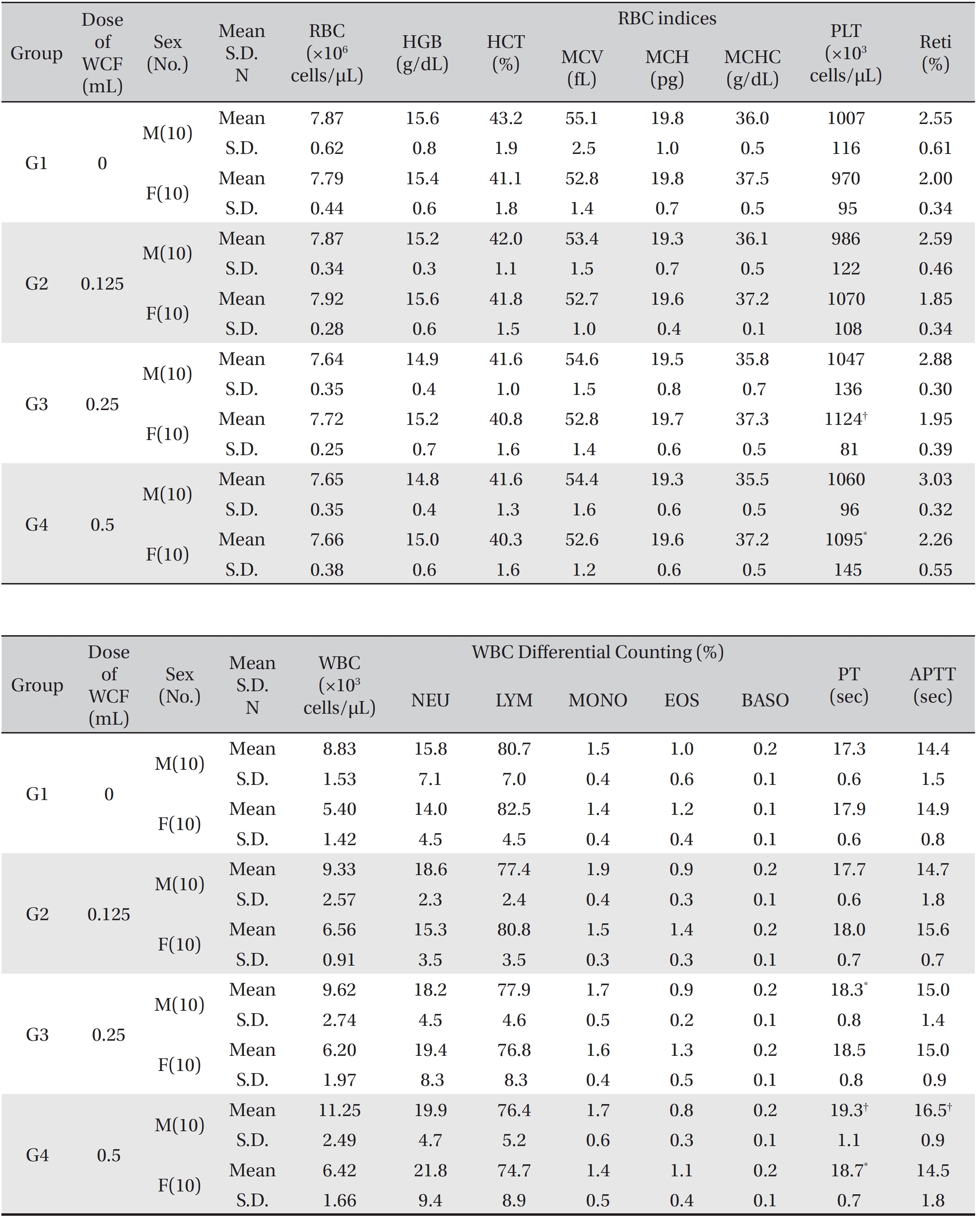
[Table. 3] Mean hematology parameters
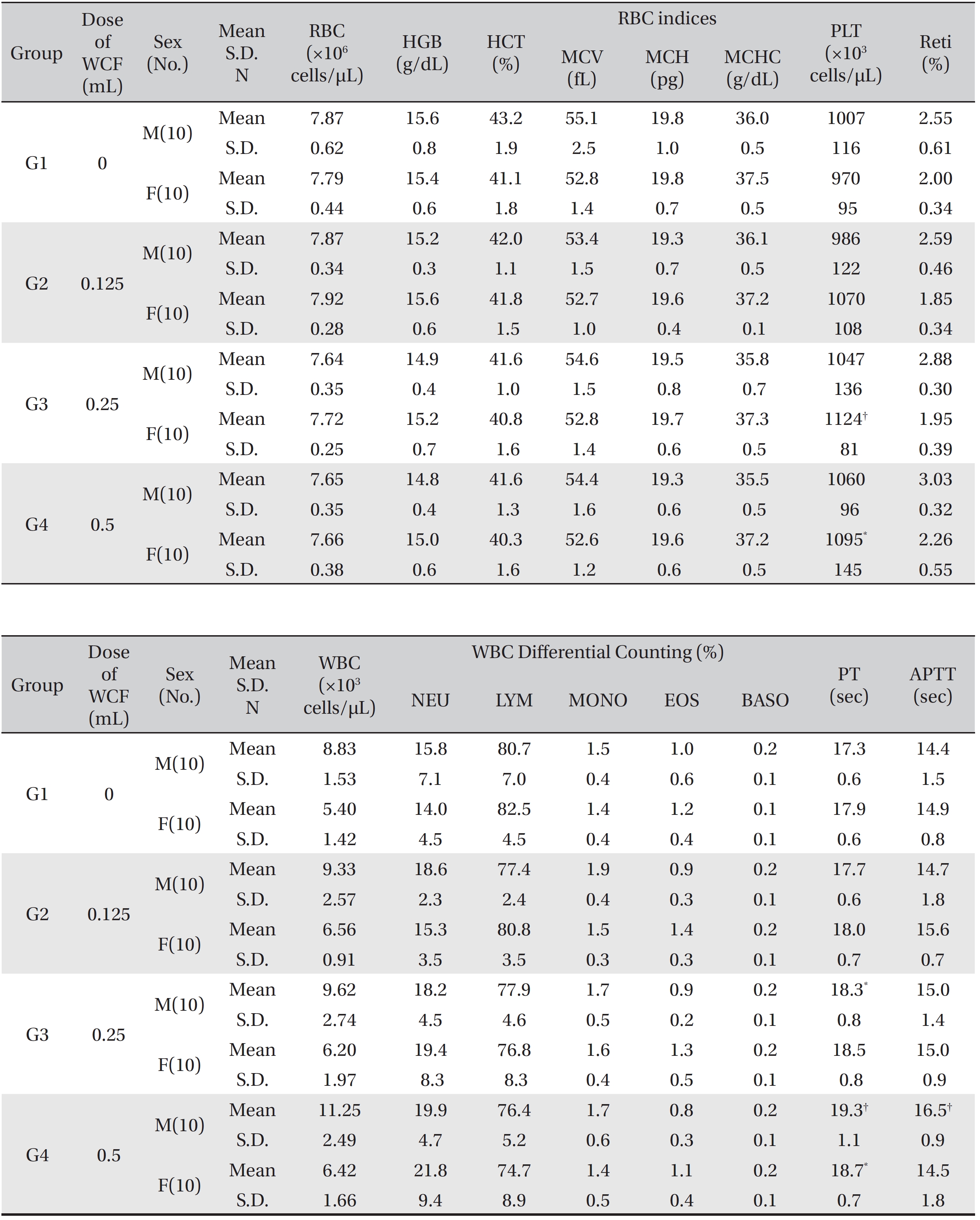
Mean hematology parameters
The hematological parameters revealed that some male rats of groups G3 and G4 showed statistically significant delays in the PT with
Several items had mean statistical significance in the clinical chemistry analyses, but they were small difference and no abnormalities was found in either the related items or the histopathological tests. In addition, no dose dependence was obvious. Therefore, the result of the clinical chemistry tests had no significant meanings from the perspective of toxicology.
After necropsy, organ weights were measured and all organs and tissues were visually inspected, and no significant changes were found compared with the control group.
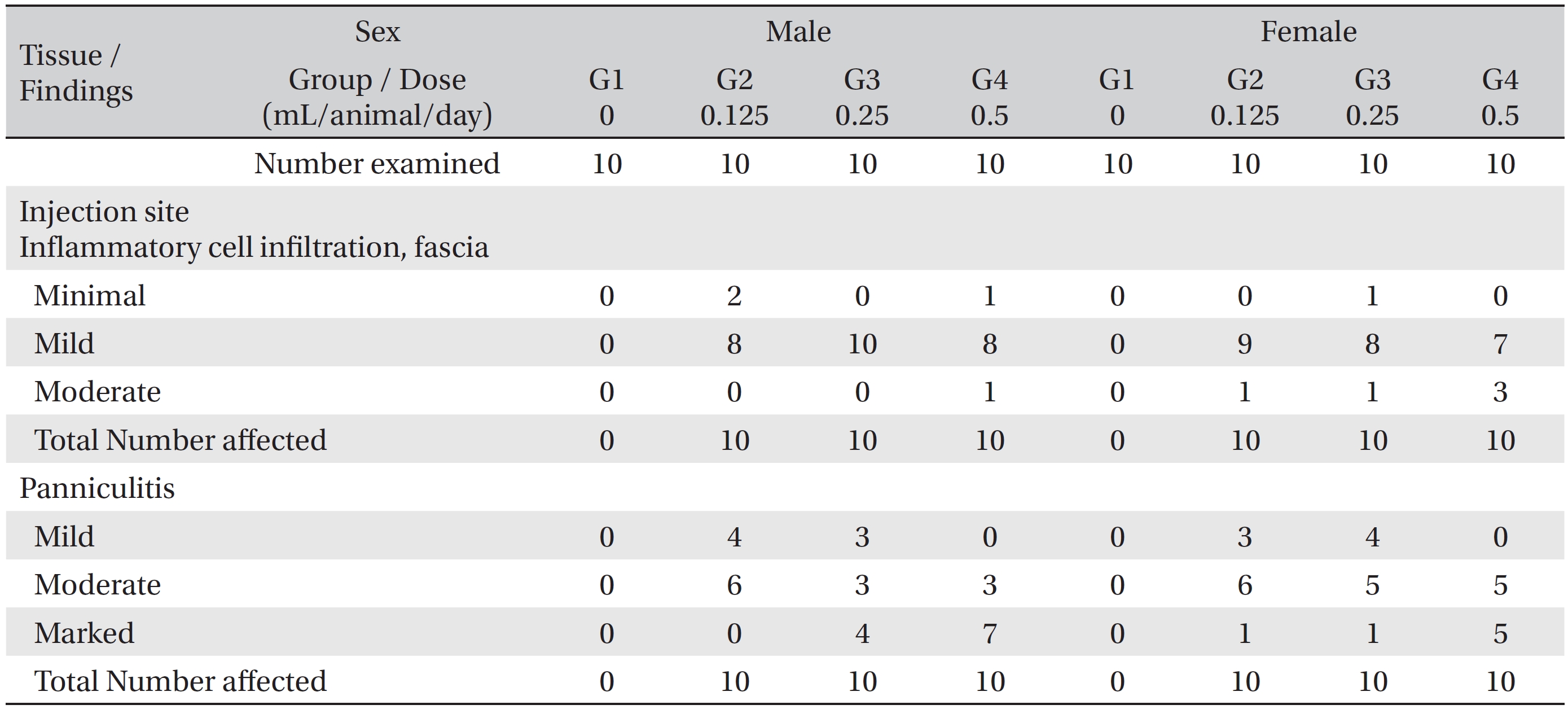
In the histopathological tests, inflammatory cell infiltration into the fascia and panniculitis in perimuscular tissues at the local injection points were observed in all animals of the experimental groups. The inflammatory cell infiltration into the fascia was mainly observed at the epimysium near the intermuscular adipose tissue of the thigh. In several cases, the infiltration was also observed in the perimysium and endomysium. The panniculitis was assumed to have been caused by the spread of WCF. The conditions were worsened by increasing the dose of WCF. However, the effects were limited to the local injection points, and no significant abnormalities were found in related organs or on related tests (Table 4). The other cases, the observed findings for other organs and tissues were thought to have occurred naturally or by chance.
Pharmacopuncture is a new form of acupuncture treatment combining acupuncture and herbal medicine. With acupuncture based on meridian theory and herbal medicine based on Qi and flavor theory, pharmacopuncture is expected to create a synergy of acupuncture and herbal medicine [1].
Nowadays, several types of research have been conducted with respect to pharmacopuncture. Other than clinical trials, two different approaches exist in experimental studies. One is an effort to broaden the field of Korean medicine by finding new herbal materials or new types of pharmacopuncture. For example, studies about Bufonis venenum [11] or
Toxicity tests are intended to examine the lethal dose or the harmfulness of new substances administered in an appropriate manner. The test should be performed in three steps, acute toxicity test (single-dose toxicity test), subacute toxicity test (four-week repeated dose toxicity test), and chronic toxicity test (three month repeated dose toxicity test) [13]. All of these tests are controlled by the toxicity testing guidelines of the Ministry of Food and Drug safety (MFDS) and evaluated against GLP standards [14].
This study is a series of toxicity tests involving a new mixture of CF. WCF is a water-soluble form of CF which was developed to increase the absorptivity of the oil form and to decrease adverse effects. Eighty male and female SD rats after 7 days of acclimation were used for the test. They were divided into four groups: Group 1 was the control group whose rats were administered normal saline solution at a dose of 0.5 mL/animal/day. Groups 2, 3 and 4 were the experimental groups whose rats were administered WCF solution at doses of 0.125, 0.25 and 0.5 mL/animal/day, respectively. For four weeks, each solution was injected into the femoral muscles of the rats, alternating from side to side.
Clinical signs, body weights, food consumptions, opthalmological examinations and urinalyses were done during the 28 days of the test. On day 29, after 18 hours of fasting, blood samples were taken for hematological analyses and clinical chemistry analyses. Then, necropsies were conducted on all animals to observe weights, as well as external and histopathological changes in body organs. All data were tested using SAS.
As a result, no deaths were observed during the four weeks of test in either the control group or the experimental groups. Irregular respiration was observed in some male rats of the 0.125, 0.25 and 0.5 mL of WCF/animal/day groups, but it was temporary and sporadic and occurred after injection. For body weights, food consumptions, opthalmological examinations, urinalyses, clinical chemistry analyses, organ weights and necropsies, no findings with toxicological significance were caused by injection of WCF in the experimental groups. On the hematological analyses, a delay of the PT was observed in male rats of the 0.25 and the 0.5 mL of WCF/animal/day groups. Finally, on the histopathological tests, inflammatory cell infiltration into the fascia (grade: minimal to moderate) and panniculitis in perimuscular tissues (grade: mild to marked) at the local injection points were observed in all animals in the experimental groups. The grade of severity was dose dependent, but these symptoms were limited to the local injection points.
According to the results, after our four-week repeated intramuscular dose test of WCF, we can draw a conclusion that WCF solution has no significant toxicological effect except for localized symptoms. Also, no observed adverse effect level (NOAEL) of WCF in male and female rats is expected at doses over 0.5 mL/animal/day. Nevertheless, this study is only a series of toxicity tests for the subacute stage. Also, the WCF solution may cause inflammatory cell infiltration or panniculitis. Further chronic toxicity studies and studies to improve symptoms must be conducted as a next step. Moreover, the harmfulness of the WCF solution in human subjects must be examined.
[Table. 4] Histopathological findings ? test substance related incidence and severity
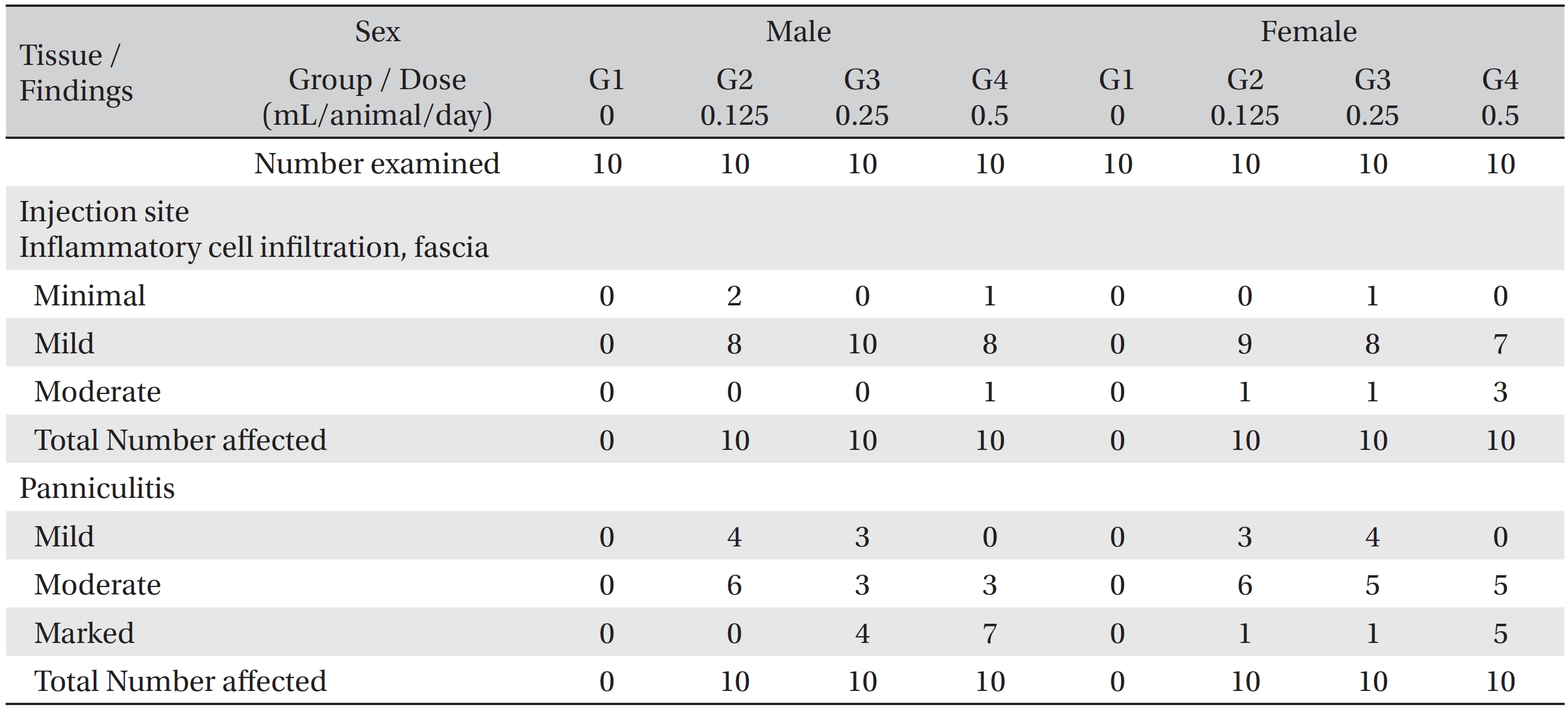
Histopathological findings ? test substance related incidence and severity
In the study, 0.125, 0.25 and 0.5 mL/animal/day injections of WCF solution did not cause any deaths. Local and dose dependent inflammatory cell infiltrations into the fascia and panniculitis in the perimuscular tissues at the local injection points were observed in all animals of the experimental groups. In this experiment, after the 4-week repeated intramuscular dose toxicity test in SD rats, the NOAEL of the WCF solution was estimated to be above 0.5 mL/animal/day for rats of both sexes.