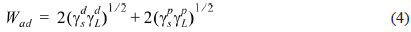Carbon fibers (CFs) have been popularly employed as an important reinforcing material in advanced composites due to their extremely high strength, high specific modulus, heat resistance, and low weight [1]. Because of these advantages, carbon fiber-reinforced plastics (CFRPs) are widely used in the aerospace [2,3], automobile [4,5], and sports industries [6]. Thermoset matrix composites have been of significant importance to the engineering community for many years. Among the available thermoset polymers, epoxy is one of the general plastics produced and consumed in large quantities, because of its strong comprehensive performance and various applications in a wide array of fields [7,8].
It is well known that the interfacial adhesion between the fiber surface and matrix in CFRPs plays an important role for almost all the mechanical properties of the composite. However, CFRPs show poor interfacial adhesion between fibers and epoxy resin, because of the inert surface and low surface energy of the CFs. This leads to poor interfacial adhesion between CFs/epoxy resin, resulting in more defects and low mechanical properties of the composites. Therefore, surface treatment should be introduced into the manufacture process of CFs to improve the interfacial adhesion between CFs and epoxy resin, finally enhancing the mechanical properties of CFRPs [9-12]. To enhance the surface properties and surface functional groups on CFs, various surface treatment methods such as electrochemical oxidation treatment [13,14], ozone oxidation treatment [15,16], plasmas treatment [17,18], and so on have been introduced. Among them, electrochemical oxidation treatment is known to be very effective for enhancing interfacial adhesion and increasing oxygen founctional groups. It is generally preferred because the treatment process is comparatively mild, and it has been widely applied in industrial manufacturing [19,20]. In order to treat the CF surface, various electrolytes have been used such as acid solutions, basic solutions, and ammonium-salt solutions. Among these, ammonium bicarbonate (ABC) is usually chosen not only because it does not cause damage to the equipment, but also because it can eliminate the residues during the heat treatment [21]. Thus, electrochemical oxidation treatment using ABC is widely used in industries to produce CFs.
In order to experimentally measure and assess the interfacial strength of CFRPs, the interfacial shear strength (IFSS) test is important. Generally three techniques have been developed to evaluate the IFSS: the fragmentation test [22,23], pull-out test [24-26], and micro-bond test [27-29]. Among these, the micro-bond test has been one of the most extensively used methods to measure the IFSS between reinforcing fibers and polymers due to the simplicity of sample preparation and IFSS measurement [30,31].
In this work, electrochemical oxidation treatment on CF surfaces was carried out to enhance the interfacial adhesion between CFs and epoxy resin. In order to evaluate the improved interfacial adhesion, the single-fiber contact angle and IFSS measurement of electrochemical oxidation treated CFs were investigated using the Wilhelmy balance method and a micromechanical test.
Un-sized polyacrylonitrile-based carbon fibers (48K; A-Company, Jeonju, Korea) were used as a reinforcing material, and ABC (Daejung Chem. Co., Korea) was used in the electrolyte. Epoxy resin (YD-128; Kukdo Chemicals & Metals Co., Siheung, Korea), diglycidylether of bisphenol-A (DGEBA), was used as the main matrix resin, and 4,4'-diaminodiphenol methane (DDM; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was used as a curing agent. The ratio of epoxy resin to the curing agent was adjusted to 1 equivalent.
2.2. Electrochemical oxidation treatment of CFs
Electrochemical oxidation treatment was carried out using a laboratory pilot appratus. Oxidation takes place in a continuous process. CFs were treated with different current densities in a range of 1 to 4 A/m2. The concentration of ABC solution, treatment time, and treatment speed were fixed at 0.5 mol, 1.4 min, and 0.7 m/min, respectively. The fibers were first immersed in an ABC solution. After oxidation treatments, the treated CFs were washed with distilled water in order to remove the adsorptive electrolyte on the CF surfaces. The electrochemical-oxidation-treated CFs, denoted as CF-AS, CF-1.0, CF-2.0, CF-3.0, and CF-4.0, were prepared at different current density.
2.3. Sample preparation and IFSS test
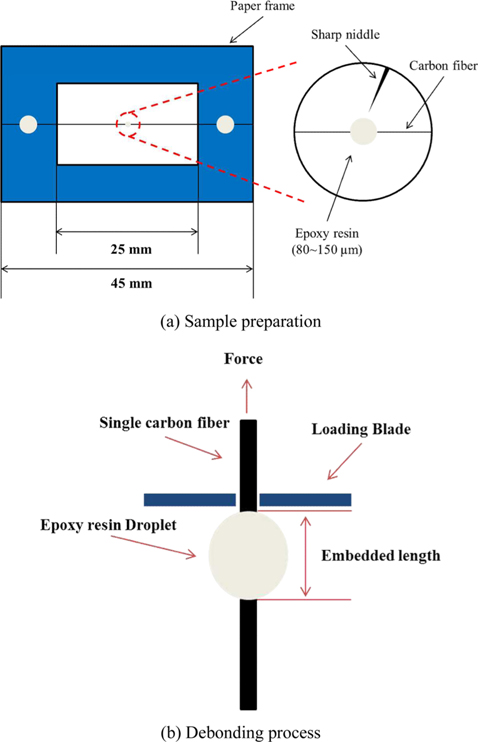
To evaluate the interfacial adhesion between electrochemical oxidation treated CFs and epoxy, a sample preparation and debonding process were conducted, as shown in Fig. 1. CFs with minimal damage were carefully selected and placed at the centerline in the middle of a paper frame, and then glued by an epoxy resin. Using a sharp needle, epoxy droplets of approximately 80–150 µm diameter were then placed on the fiber. The epoxy resin was allowed to cure at room conditions for 72 h to reach full strength.
In the traditional microbond test method, two blades are fixed so that a fiber can be pulled out from a droplet of matrix. Several significant parameters, including embedded fiber length, fiber diameter, and the peak pull-out force, are measured. At this moment, assuming that the measured force is equal to a shearing force that is applied to the whole interface and distributed evenly, the IFSS is then calculated using the following eq (1):
where F is the peak pull-out force (N), D is the fiber diameter (µm), and L is the embedded fiber length (µm) in the matrix.
2.4. Measurement of wettability and surface energies
Single-fiber contact angle measurements of electrochemical oxidation treated CFs were carried out using the Wilhelmy balance method. The dynamic contact angle is measured using two solutions, distilled water (γ = 72.8 mN/m, , and , Ström) and diiodomethane (γ = 50.8 mN/m, , and , Donnet). Where γ is the surface tension of the wetting liquide, is the dispersion component of the wetting liquide, and is the polar component of the wetting liquide. By using two testing liquids, the surface free energy of the CFs can be calculated according to the Wilhelmy balance method [32], as described in eq (2):
where F is the measured force, P is the wetted perimeter of the fiber at the liquid/air interface, γ is the surface tension of the wetting liquid, cosθ is the contact angle of the fiber surface, W is the weight of the fiber, ρ is the density of the wetting liquid, g is the gravitational acceleration, y is the immersion depth, and A is the cross-sectional area of the fiber. W is taken prior to measurement, and the buoyancy force is zero at the immersing interface. Therefore, eq (3) can be modified as:
The solid surface tensions of the investigated CFs and epoxy matrix were calculated from the measured contact angle values according to the work of adhesion (
where γs is the surface energetic of the CFs listed in Table 1, γL is the surface energetic of the epoxy matrix used and and [36].
The CF surface and microfailure modes of the microdroplet were studied by field emission scanning electron microscopy (SUPRA 40VP; Carl Zeiss, Germany). After applying Os coating, measurements were taken at an acceleration voltage of 2 kV. Surface topographical images were obtained by atomic force microscopy (AFM; Park Systems Corp., Suwon, Korea). In this analysis the tapping mode was employed with a scanning scope of 3 µm. The infrared spectra of the electrochemical oxidaition treated CFs were obtained by Fourier transform infrared spectroscopy (FT-IR; Nicolet iS 10, Thermo, USA) at 4000–500 cm−1 wavelengths. Chemical components and the relative content of functional groups on the CF surface were analyzed through X-ray photoelectron spectroscopy (XPS; PHI 5000 Versa Probe II, ULVAC-PHI; Chigasaki, Japan). The single-fiber contact angle was measured using a Krüss K-100SF (Krüss GmbH Ltd., Hamburg, Germany) with 1 µg weight resolution. A tensile test of the fiber was carried out on a Favigraph semiautomatic device (Textechno Company, Mönchengladbach, Germany). The gauge length of the fiber was 20 mm and the draw-off clamp speed was set at 1 mm/min. A micro-bond test for single-fiber was carried out on a universal material testing machine (Lloyd, UK) with a constant speed of 0.1 mm/min.
3.1. Surface wettability of CFs
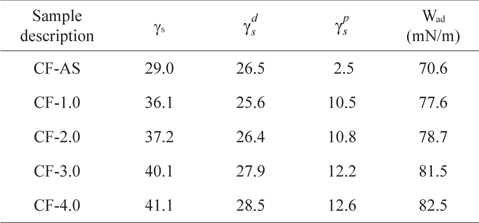
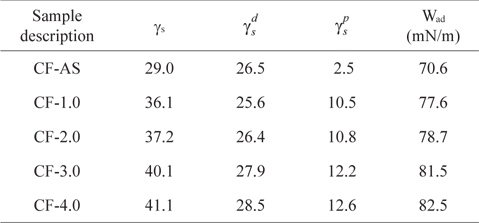
Table 1 shows the dispersive and polar components of the surface free energies and the work of adhesion for the untreated and electrochemical oxidation treated CFs. The surface free energy and work of adhesion of the CFs increased after electrochemical oxidation treatment. If the value of the polar component after the treatment is high, many hydrophilic groups such as hydroxyl (-OH), carbonyl (C=O), carboxyl (-COOH), and amine (-NH2) can exist on the fiber surface [37]. Meanwhile, an increase of dispersive components is possible due to the increase of specific surface area due to the roughness change after the treatment. Therefore, from this result it is seen that the surface of the electrochemical-oxidation-treated CFs was significantly changed in terms of both dispersive and polar components, resulting in improvement of the surface adhesion with matrices.
. Surface energetics of electrochemical-oxidation-treated CFs as function of current densities
3.2. Surface properties of CFs
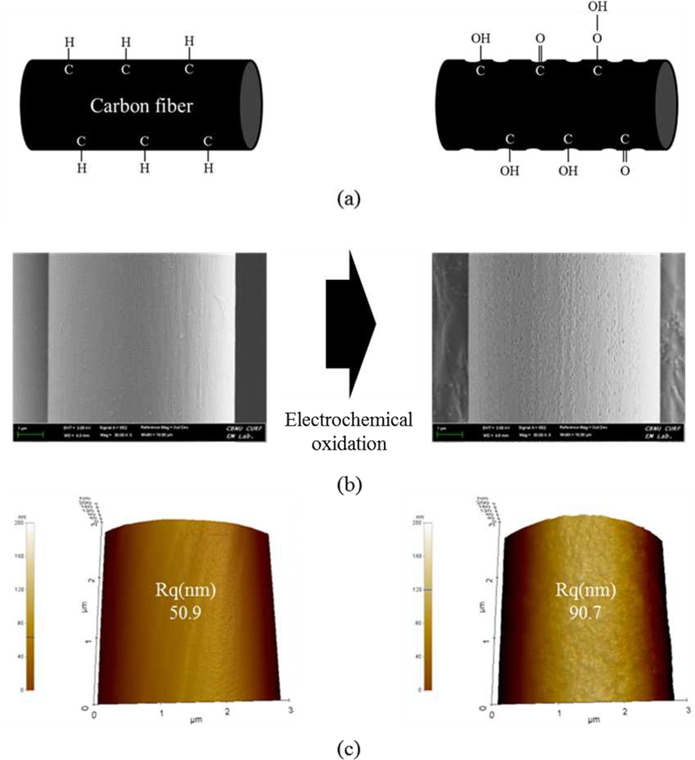
The effects of the electrochemical oxidation treatment on the surface morphology of CFs were analyzed with scanning electron microscopy (SEM) and AFM images. As shown in Fig. 2, the surface of CF-AS showed a characteristic flat topography. After electrochemical oxidation treatment, the surface is uniformly rough with many deep grooves. In addition, after electrochemical oxidation, the value of the root mean square average roughness (Rq) was increased from 50.9 to 90.7 µm. The electrochemical oxidation reaction generally damages the CFs and leads to deep grooves and edges on the CF surface, and this is believed to account for the greater roughness. This increase in surface roughness should be advantageous for mechanical properties of CFPRs because a rough surface would lead to good mechanical interlocking and enhance the interfacial bonding strength between the CFs and epoxy resin.
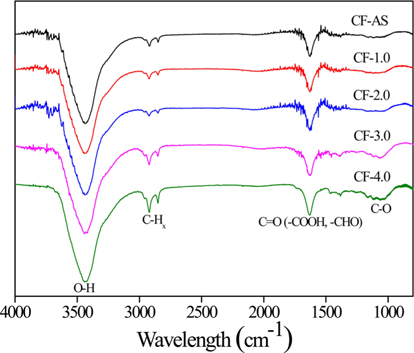
Besides enhancing mechanical interlocking between CFs and epoxy resin, electrochemical oxidation treatment introduced oxygen-containing functional groups and thereby increased the surface polarity. Fig. 3 shows the FT-IR spectra of the electrochemical-oxidation-treated CFs as a function of current densities. The oxygen functional groups including -OH (3500 cm−1), C=O (1634 cm−1), and C-O (1082 cm−1) are improved with increasing current density. The -OH or -COOH groups are capable of forming covalent interfacial bonds in the cross-linked polymer adhesive. These bonds couple to the fiber and optimally transfer stresses between the matrix and the fiber, thereby enhancing the interfacial adhesion [38,39]. It is wellknown fact that the increase in oxygen functional group after a surface treatment can lead to the enhancement in mechanical properties [40].
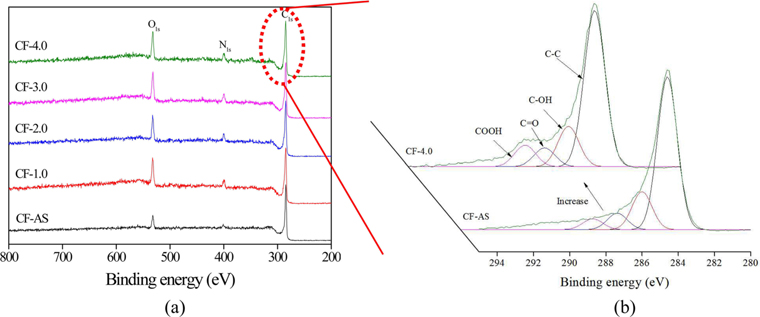
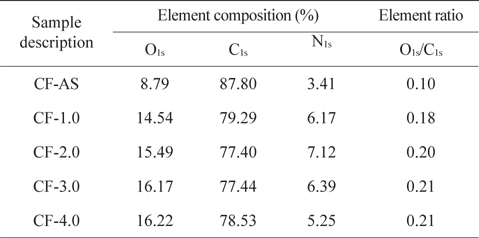
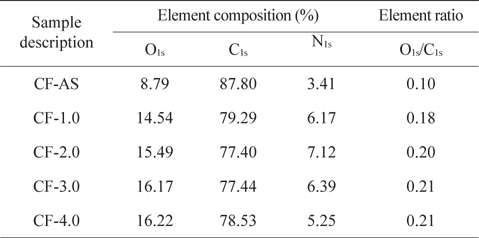
XPS survey spectra of the electrochemical-oxidation-treated CFs and analysis results are shown in Fig. 4 and Table 2. Fig. 4a shows the carbon (C1s, BE [binding energy] = 284 eV), oxygen (O1s, BE = 532 eV), and nitrogen (N1s, BE = 400 eV) peaks. It was found that C1s peak decreased with increasing current density, while the O1s peak increased. As can be seen from the XPS sub-peak analysis, the content of oxygen functional groups, such as C-OH (BE = 285.9–286.2 eV), C=O (BE = 287.3–287.6 eV), and COOH (BE = 288.6–288.7 eV), increased with current density. The O1s/C1s ratios and elemental content on the surfaces of CFs by the electrochemical oxidation treatment are shown in Table 2. The increased degree of oxygen content on the CF surface can be confirmed by the O1s/C1s. All treated CFs showed higher oxygen content than that of the CF-AS. This indicates that the electrochemical oxidation treatment effectively introduced a large amount of oxygen containing functional groups. This in turn improves the interfacial adhesion between CFs and epoxy resin in a composite system.
X-ray photoelectron spectroscopy surface element concentrations of the untreated and treated CFs
3.3. Mechanical properties of CFs
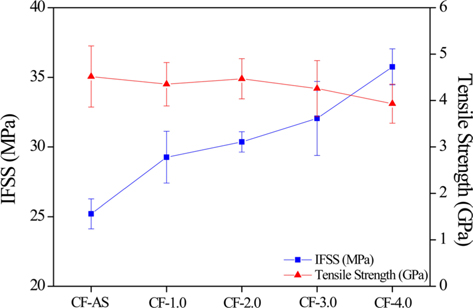
IFSS and tensile strength values of untreated and surface treated CFs are shown in Fig. 5. The tensile strength value of the CFs slightly decreased (86% of original strength) with increasing current densities. This showed that the chemical etching effect on the CF surface in the process of electrochemical oxidation could degrade the mechanical properties of the CFs. In contrast, the IFSS values of the CFs increased (144% enhanced) with increasing current densities. The roughness of the CF surfaces could result in an increase of the surface contact area and enhance the physical bonding between the CFs and epoxy resin, and the increased content of oxygen functional groups could improve the interfacial adhesion between the CFs and epoxy resin, as confirmed by the work of adhesion. Therefore, because of the increase in the amount of oxygen functional groups and the roughness of the CF surface during the electrochemical oxidation treatment process, the mechanical interfacial strength of the CFRPs was dramatically enhanced.
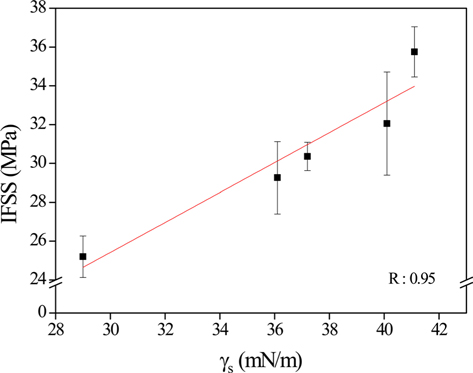
The trend of the IFSS results is similar to that of the surface free energy (γs). In fact, the correlation of IFSS with γs is almost linear for all samples with different current densities, as shown in Fig. 6. Therefore, γs is one of main factors affecting the adhesion between the fibers and the epoxy matrix.
3.4. Fracture morphological structure after IFSS test
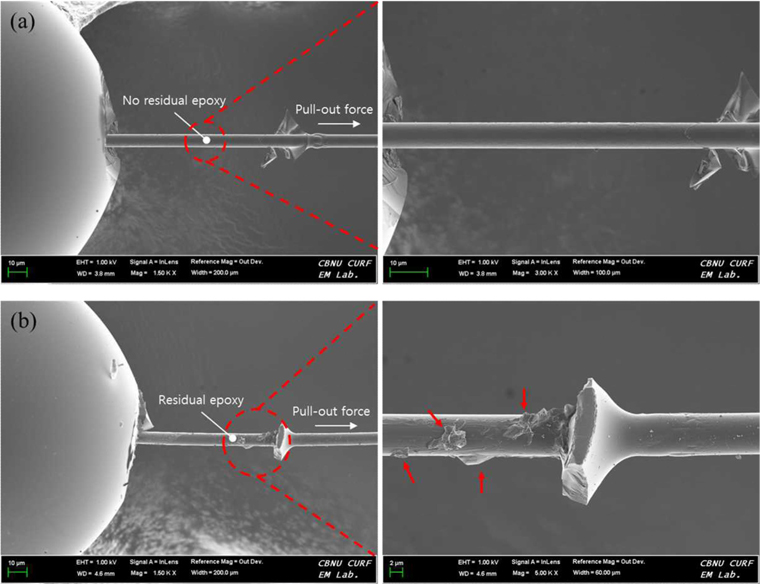
Fig. 7 shows SEM images for the untreated and electrochemical oxidation treated CF/epoxy composites after the micro-bond test for CF-AS and CF-4.0. As shown in the figure, the morphology of the untreated and electrochemicaloxidation-treated sample images differs. In Fig. 7a, no residual epoxy resin in the de-bonding zone was observed for the untreated CFs while a large amount of residual epoxy resin remained in the de-bonding zone for the electrochemicaloxidation-treated CFs. This showed that the electrochemical oxidation treatment on CFs provided better IFSS due to increased surface roughness and the introduction of oxygen functional groups.
In this study, electrochemical oxidation treatment with different current densities was performed. After electrochemical oxidation treatment, the adhesion strength between CFs and epoxy resin predominantly increased. The electrochemical oxidation on the CF surface improved the IFSS of the composites due to the introduction of oxygen functional groups and an increase in roughness, attributed to the polar and dispersive component on the CF surface, resulting in enhanced interfacial adhesion between the CFs and epoxy resin in this composite system.