In general, soybeans [Glycine max (L.) Merril] have been consumed as an important protein source to complement grain protein in Asian countries. However, they are also enriched with isoflavones, anthocyanins, saponins, lipids, and oligosaccharides (Kim et al., 2011). The several soy-foods, such as cheonggukjang, doenjang, douche, temphe, and tofu, are being prepared from soybeans through fermentation process.
Among several soybean varieties, black soybean (BS) is increasingly sought after in food and medicinal industries because of their various beneficial effects (Lee et al., 2012). The beneficial properties of BS are due to the many phytochemicals present in the crop, including isoflavone, flavanol, flavan-3-ol, anthocyanin, and saponin (Lee et al., 2012; Lee et al., 2014). BS is very much popular in Korea and are used to prepare several different traditional fermented foods, including meju (soybean cake), cheonggukjang (soybean cook), kanjang (soybean sauce), and doenjang (soybean paste). In particular, cheonggukjang is manufactured in a traditional way in homes using different types of processes, depending on the region: thus its physicochemical and functional properties vary due to differences in soybeans, microorganisms, and fermentation time (Nam et al., 2012).
In raw soybeans, isoflavones are present in four chemical forms: malonylglycosides (70-80%), acetylglycosides (5%), glycosides (25%), and aglycones (2%) (Lee et al., 2011). Isoflavones conjugated glycoside are converted to aglycones under acidic or alkaline conditions or by the action of -glycosidase. Importantly, the aglycone forms show greater potential for absorption in the intestine than the glycoside forms (da Silva et al., 2011). Thus, incorporation of β-glycosidase has attempted to increase the content of isoflavone- aglycones in cheonggukjang in several studies (Yang et al., 2006; Cho et al., 2011). Moreover, some studies reports that total phenolic and isoflavone-aglycone contents increased, depending on whether antioxidant activities increased after cheonggukjang fermentation (Cho et al., 2009; Hu et al., 2010). Recently, we reported that the total phenolic and isoflavone- aglycone contents were enhanced during cheonggukjang fermentation made with two wild varieties of black soybeans (Hwang et al., 2013) and brown soybean (Shin et al., 2014) using a potential probiotic Bacillus subtilis CSY191 (Cho et al., 2009; Cho et al., 2011).
In this study, the antioxidant activities in cheonggukjang made with four BS cultivars including Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong by the potential probiotic B. subtilis CSY191 were investigated. Moreover, the possibility of antioxidant enhancing effect during cheonggukjang fermentation made with BS cultivars that may be related to the total phenolic contents and isoflavone compositions of this product were also investigated.
Four BS cultivars, including Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong, were harvested in 2012 and were procured from the National Institute of Crop Science (NICS) of the Rural Development Administration (RDA) in Miryang, Korea. The collected BS samples were packaged in labeled vacuum pouches to prevent their degradation. The potential probiotic Bacillus subtilis CSY191 previously isolated from Korean traditional soybean paste (doenjang), were used as the starter organism (Cho et al., 2011). The twelve standard isoflavones were purchased as described previously (Hwang et al., 2014). Glacial acetic acid, Folin-Cicalteu phenol reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt (ABTS), potassium persulfate, ferric chloride, sodium acetate, 2,4,6-tripyridyl-s-triazine (TPTZ), were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). HPLC-grade H2O, methanol, and acetonitrile were purchased from Fisher Scientific Korea ltd. (Gangnam-gu, Seoul, Korea). All other reagents were of analytical grade.
Approximately 1,000 g of BS from each cultivar were separately washed and soaked with 2.5 volume tap water at 25±2℃ for 12 h, and steamed for 15 min at 121±1℃. The steamed HBS were left to stand for 1 h at 40±2℃ to cool down. After that, cooked BS were inoculated with 5.0% (w/w) strain CSY191 (7.65 log cfu/mL) and fermented for 48 h at 37±2℃ in an incubator and sampled at 0 and 48 h. The method was adapted as previously described (Hwang et al., 2013).
One gram of Cheonggukjang was mixed with 9 mL of 0.85% NaCl solution and the diluted suspension (0.1 portions) was spread on a TSA plate. The plate was incubated at 37±1℃ for 48 h and after which colony counts were carried out. A 10 g portion of the cheonggukjang of BS samples was dissolved in 90 mL of distilled water at room temperature for 12 h and was filtered through Whatman No. 4 filter paper (Whatman International, Ltd., Maidstone, England). The residue pH was measured by pH meter (MP 200, UK). Moreover, the β-glucosidase activity of the Cheonggukjang crude extract was measured as previously described (Hwang et al., 2013).
The four BS Cheonggukjang samples isoflavone were extracted and analyzed by HPLC according to previously described method (Hwang et al., 2013).
A method based on gallic acid equivalents (GAE) was used to quantify the TPCs of 50% methanol extract of four BS Cheonggukjang samples according to Cho et al. (2009).
The 50% methanol extracts of BS Cheonggukjang (0.2 mL) were prepared and mixed with 0.8 mL of 1.5×10-4 mM DPPH methanolic solution. The mixture was vortexed vigorously and allowed to stand for 30 min at room temperature in the dark. The absorbance of the mixture at 517 nm was determined using a spectrophotometer. The scavenging activity was expressed as a percentage using the following formula: DPPH radical scavenging activity (%) = (1-absorbance of sample/absorbance of control)×100. The DPPH radical scavenging activity of Cheonggukjang extracts was carried out according to Kim et al. (2013) that was adapted from Cho et al. (2011).
ABTS+ was dissolved in methanol to a finial concentration of 7 mM. This radical cation was produced by reacting the ABTS+ stock solution with 2.45 mM potassium persulfate (final concentration) and by leaving the mixture for 12-16 h until the reaction was complete and the absorbance was stable. The ABTS+ stock solution was diluted in ethanol to an absorbance of 0.7±0.02 at 734 nm. After adding 0.9 ml of the diluted ABTS+ solution to 0.1 mL of the sample and mixing them, the absorbance was taken 3 min later. The ABTS radical scavenging activity (%) of four cultivars of Cheonggukjang extracts was expressed as a percentage using the following formula: ABTS radical scavenging activity (%) = (1-absorbance of sample / absorbance of control)×100 (Kim et al., 2014).
1.5 mL of working ferric reducing/antioxidant power (FRAP) reagent pre-warmed to 37℃ was mixed with 50 μL of the test samples and standards. After vortexing, the mixture absorbance was read at 593 nm against a reagent blank. The assay was conducted at 37℃ for 15 min. The FRAP assay was conducted according to Choi et al. (2012).
Data were expressed as the means SD (standard deviation) of three replicates. The results were subjected to analysis of variance followed by the Tukey's multiple range tests at p<0.05 using the SAS program (Version 9, USA).
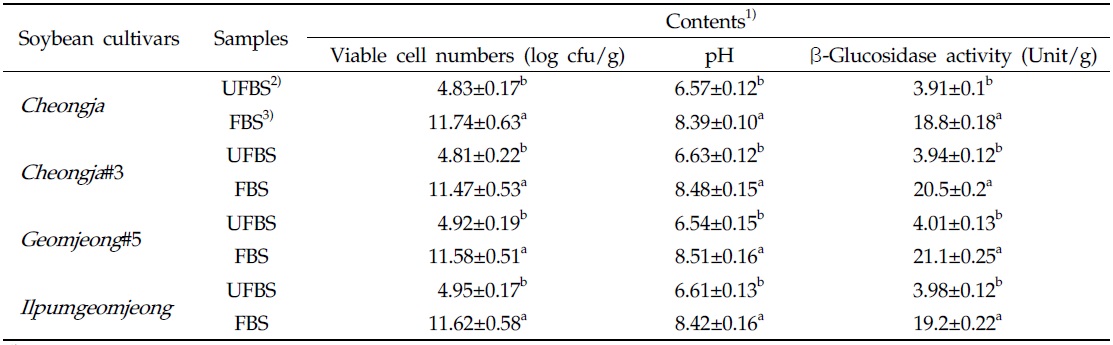
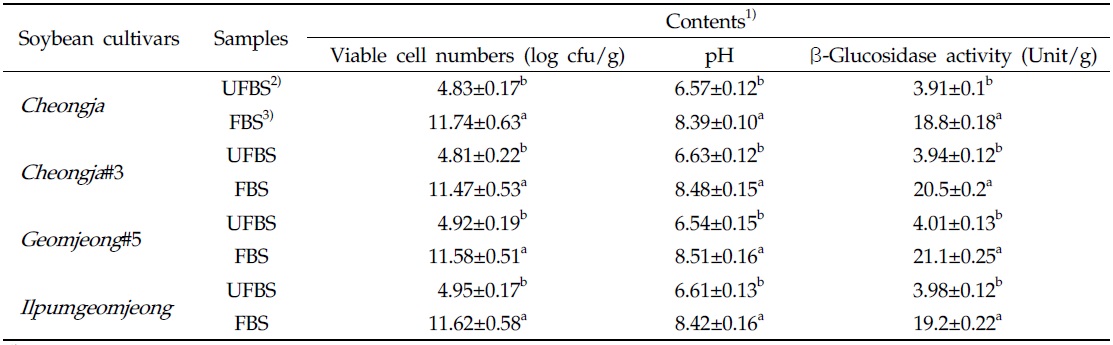
The viable cell numbers, pH, and β-glycosidase activities in each cheonggukjang made with four BS cultivars were increased during fermentation (Table 1). As the results, the bacterial cell numbers in fermented cheonggukjang made with BS of Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong cultivar were increased to 2.43-, 2.38-, 2.35-, and 2.35-fold, respectively. In addition, the β-glucosidase activities in cheonggukjang made with BS of Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong cultivars were increased to 4.8-, 5.2-, 5.26-, and 4.82-fold by the end of fermentation (48 h), respectively. In fact, the pH of fermented cheonggukjang made with BS from all four cultivars was raised approximately to 8.39 to 8.51. The viable cell numbers and β-glycosidase activities were markedly increased during cheonggukjang fermentation in previous studies (Yang et al., 2006; Cho et al., 2011; Hwang et al., 2013; Shin et al., 2014), which is a very good agreement with the current study.
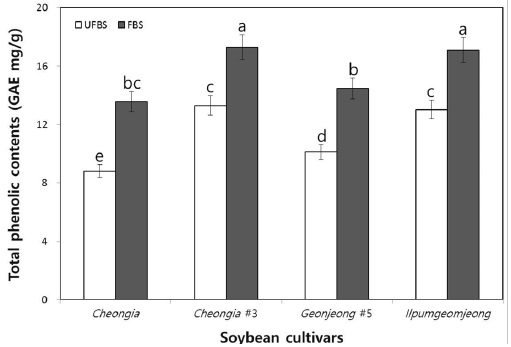
The change in the TPCs in cheonggukjang made with BS of Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong cultivars is shown in Fig. 1. The TPCs in each fermented cheonggukjang made with BS from all cultivars were increased than that of the unfermented BS. In particular, the TPCs in fermented cheonggukjang made with BS were increased to 1.53-, 1.3-, 1.43-, and 1.31-folds by the end of fermentation (48 h) for the Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong cultivars, respectively (Fig. 1). Phenolics are usually found in conjugated forms through hydroxyl groups with sugars and glycosides in plant materials (Juan & Chou, 2010). Catalyzing the release of the total phenolic contents from the BS during fermentation may thus lead to an increase in the content of those compounds, as shown in Fig. 1. Our previous study reported that the TPCs in fermented cheonggukjang made with brown soybean (Galmi) and wild BS cultivars (Seoritae and Seomoktae) were increased in amount with potential probiotic Bacillus subtilis CSY191 by the end of fermentation (Hwang et al., 2013; Shin et al., 2014). These results suggested that Bacillus subtilis CSY191 is a strong potential candidate strain for the biotransformation of soybean biopolymers into beneficial phenolics during cheonggukjang fermentation made with wild or breeding soybean seeds. Meanwhile, some studies reported that the total phenolic content increased during soybean fermentation in foods, such as cheonggukjang and natto (Cho et al., 2009; Cho et al., 2011; Shon et al., 2007).
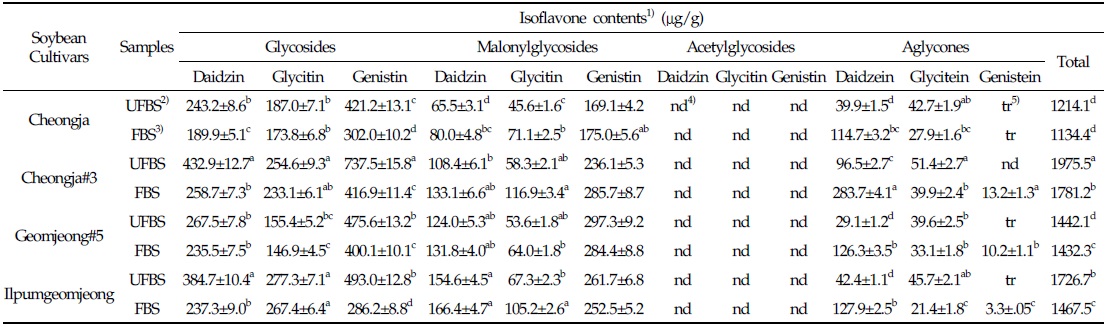
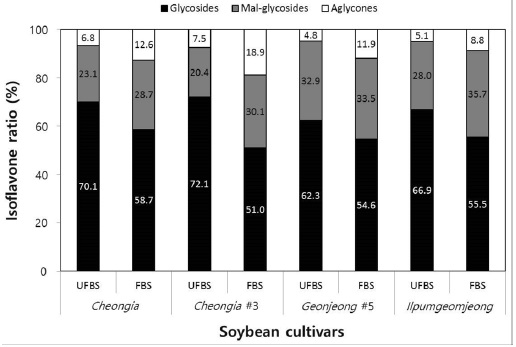
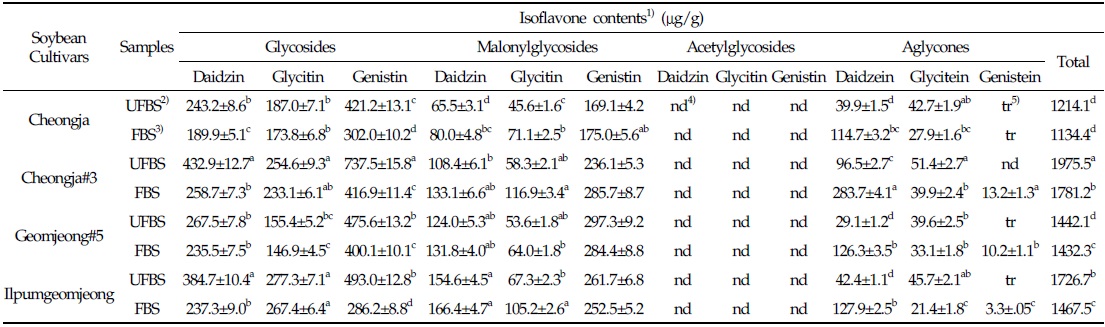
In the case of chenoggukjang made with BS of Cheongja cultivar, the isoflavone-malonylglycoside and-aglycone contents increased throughout fermentation to approximately 1.24- and 1.85-fold relative to their starting amounts (23.1% and 6.8%, respectively), but the isoflavone-glycoside contents decreased from 70.1 to 58.7% at the end of fermentation. Similarly, the levels of isoflavone-malonylglycoside and -aglycone in the chenoggukjang made with BS of Cheongja#3, Geomjeong#5, and Ilpumgeomjeong cultivars increased throughout fermentation to approximately 1.47- and 2.52-fold, 1.02- and 2.48-fold, and 1.27- and 1.72-fold relative to their starting amounts (20.4 and 7.5%, 32.9 and 8.8%, and 28.0 and 5.1%), but the isoflavone-glycoside contents decreased from 72.1 to 51.0%, 62.3 to 54.6%, and 66.9 to 55.5%, respectively, at the end of the fermentation (Fig. 2). In particular, daidzin of the glycoside type decreased from 432.9 μg/g to 258.7 μg/g and the corresponding daidzein of the aglycone type increased from 96.5 μg/g to 283.7 μg/g in chenoggukjang made with BS of Cheongja#3 cultivar at the end of fermentation (Table 2). In fact, the total amount of isoflavone was higher in unfermented and fermented (chenoggukjang) BS of Cheongja#3 cultivar than those of the other BS cultivars examined. However, it is apparently shown that the isoflavone-glycosides decreased, while the isoflavone-aglycones increased during cheonggukjang fermentation made with all the four BS cultivars (Fig. 3A-H). These results suggested that the glycoside type isoflavone content daidzin, glycitin, and genistin concentrations were decreased in chenoggukjang made with all BS cultivars, while the aglycones type isoflavone content daidzein, glycitein, and genistein concentrations were increased at the end of fermentation. In addition, the malonylglycosides concentrations were also increased in the fermentation of BS chenoggukjang than that of the unfermented BS. The content and composition of these isoflavones vary in soybean foods depending on the soybean varieties and processing techniques used, such as fermentation. It has been reported that the isoflavone levels in soybean-containing foods, such as tofu, douchi, and cheonggukjang, decrease depending on the processing conditions (Yang et al., 2006; Cho et al., 20011; Coward et al., 1998; Prabhakaran et al., 2006). Jang et al. (2006) reported that the total isoflavone content in raw soybeans was 2.87 μg/g, which decreased by approximately 50% during cooking prior to cheonggukjang fermentation. In a related study, Yang et al. (2006) reported that the total isoflavone content decreased from 1,055 μg/g (0 h) to 870 μg/g (36 h) during cheonggukjang fermentation by B. subtilis. Meanwhile, Cho et al. (2011) reported that the total isoflavone contents in cheonggukjang fermentation decreased approximately 64% from an initial 2923.21 μg/g to 1051.59 μg/g after 60 h of fermentation. Hwang et al. (2013) reported that the total isoflavone content in cheonggukjang made with wild soybeans was decreased by 13.15% and 8.3% for the Seoritae and Seomoktae cultivars using Bacillus subtilis CSY191. In this study, the total isoflavone content decreased by approximately 6.6, 9.84, 0.7, and 15.1% after fermentation processing in BS of Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong cultivars at the end of fermentation (48 h), respectively (Table 2).
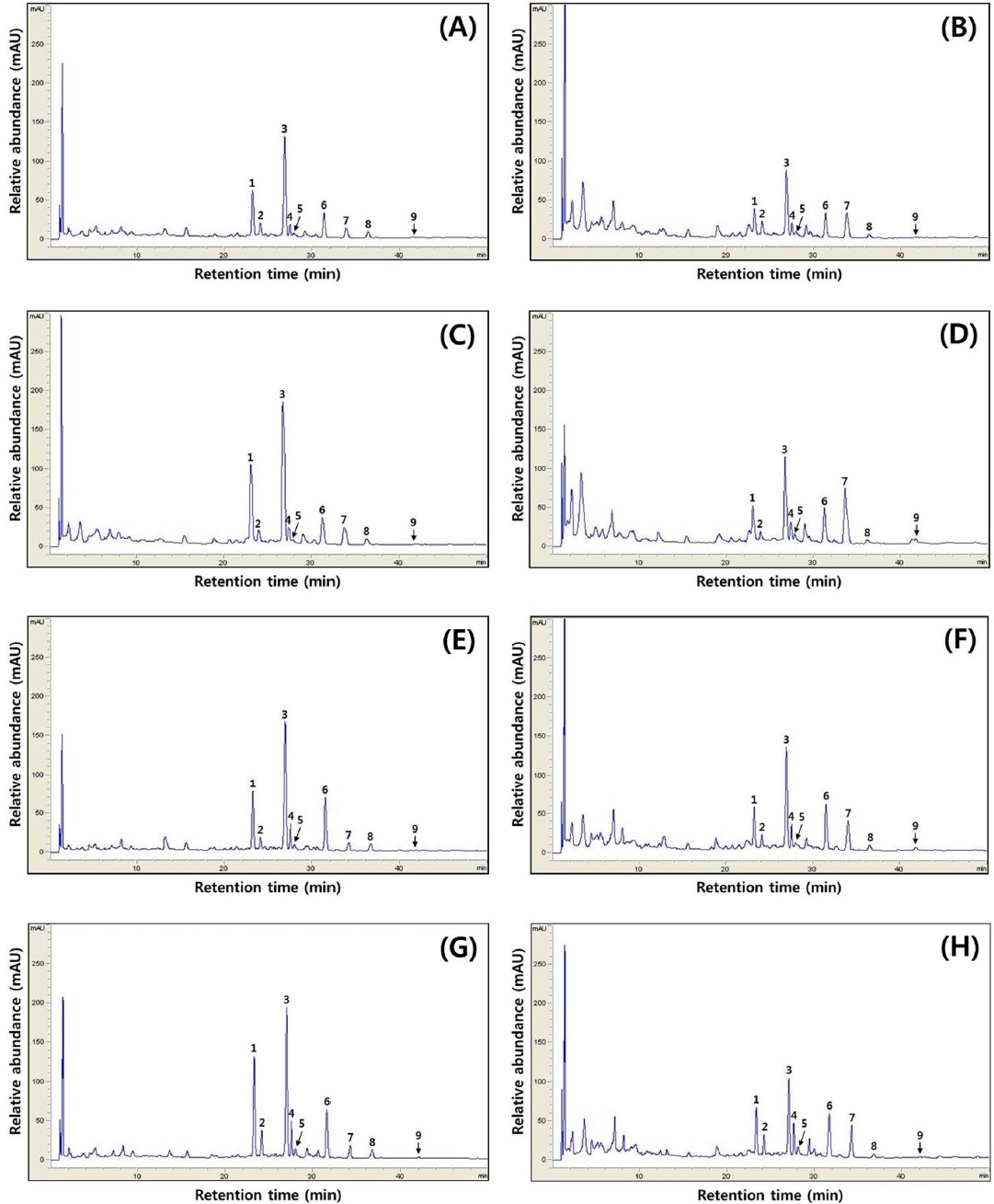
[Fig. 3.] Typical HPLC chromatograms of isoflavones. HPLC chromatogram of isoflavone extract in cheonggukjang made with black soybean of (A) Cheongja cultivar according to fermentation period (0 h), (B) Cheongja cultivar according to fermentation period (48 h), (C) Cheongja#3 cultivar according to fermentation period (0 h), (D) Cheongja#3 cultivar according to fermentation period (48 h), (E) Geomjeong#5 cultivar according to fermentation period (0 h), (F) Geomjeong#5 cultivar according to fermentation period (48 h), (G) Ilpumgeomjeong cultivar according to fermentation period (0 h), and (H) Ilpumgeomjeong cultivar according to fermentation period (48 h). 1, diadzin; 2, glycitin; 3, genistin; 4, malonyldaidzin; 5, malonyl glycitin; 6, malonyl genistin; 7, daidzein; 8, glycitein; and 9, genistein.
In general, most isoflavones in soybean are present in glycoside form, and they are converted into aglycones during fermentation by microbial β-glycosidase activity (Velioglu et al., 1998; Yang et al., 2006; Cho et al., 2011). It is important to note that the levels of isoflavone-aglycones and the β-glycosidase activity increased and isoflavone-glycosides decreased during cheonggukjang fermentation by the potential probiotic B. subtilis CS90 (Cho et al., 2011; Hwang et al., 2013). In this study, we found that the starter potential probiotics B. subtilis CSY191 had the effect of increasing the β-glycosidase activity, and the algycone contents increased at the end of fermentation. In contrast, Yang et al. (2006) reported that the addition of B. subtilis had no effect on β-glycosidase activity, and the aglycone contents did not increase during cheonggukjang fermentation.
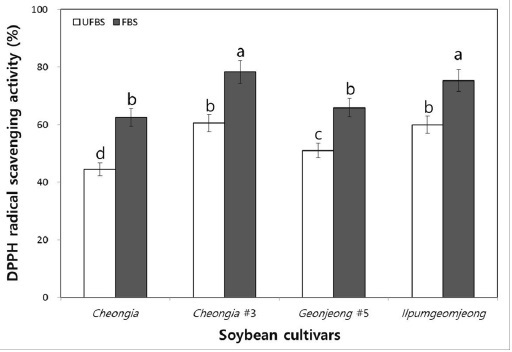
To examine the hydrogen donating activity, the DPPH radical scavenging activity of the unfermented black soybean (UFBS) and fermented black soybean (FBS) were tested. As the result, the DPPH radical scavenging activities of chenoggukjang made with different BS cultivars were increased about to 1.4-, 1.3-, 1.3-, and 1.25-folds at the end of fermentation, respectively (Fig. 4). This result suggested that the hydrogen donating activities of BS were increased after chenoggukjang fermentation. Importantly, the chenoggukjang made with BS of Cheongja#3 cultivars has shown the greater DPPH radical scavenging activity (78.3%) than those of the chenoggukjang made with other BS cultivars in this study.
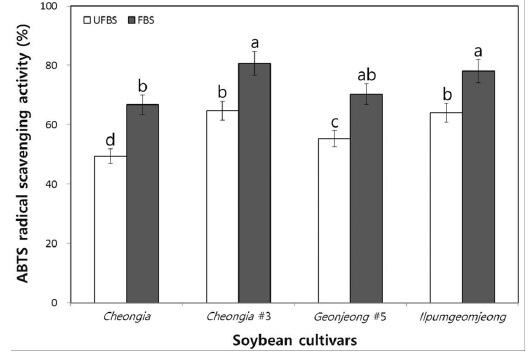
Moreover, to determine the hydrogen-donating antioxidants and chain-breaking antioxidants, the ABTS radical scavenging ability of chenoggukjang made with UFBS and FBS were tested. The levels of ABTS radical scavenging activity in chenoggukjang of Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong was increased about to 1.35-, 1.25-, 1.27-, and 1.22- folds at 48 h of fermentation (Fig. 5). However, like DPPH radical scavenging activity, the chenoggukjang of Cheongja#3 has shown the greater ABTS radical scavenging activity (80.64%) than those of the other chenoggukjang examined in this study.
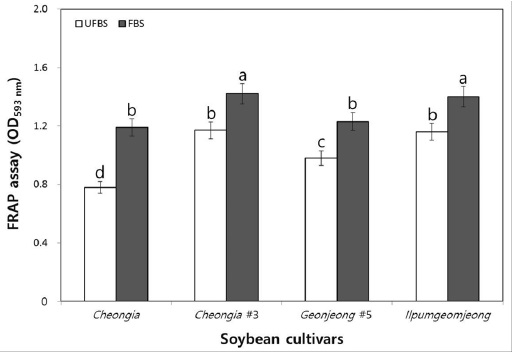
The FRAP assay directly measure the total antioxidant power of plant extracts. Thus, we further determine the antioxidant activity of the unfermented and FBSs. In fact, the chenoggukjang of Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong, the values resulting from the FRAP assay of the fermented soybeans increased about to 1.52-, 1.21-, 1.25-, and 1.2-fold at 48 h fermentation, respectively (Fig. 6). Importantly, like DPPH and ABTS radical scavenging activity, the FRAP assay value (1.42) of chenoggukjang made with BS of Cheongja#3 cultivar was greater than those of the other chenoggukjang examined.
Several studies revealed that phenolic compounds were responsible for the antioxidant activity in fruits, vegetables, and grains (Velioglu et al., 1998; Kwak et al., 2005). Pratt (1980) reported that the combined isoflavones and phenolic acids account for nearly all the in vitro antioxidant activity of soybean and soy product. In fact, the TPCs were measured as an overall indicator of the contents of these molecules with antioxidant properties (Slavin et al., 2009). It was reported that methanol extract of cheonggukjang exhibited radical-scavenging activity of 69-87% and total phenolic contents of 0.13-0.27 mg/g (Shon et al., 2007). Interestingly, fermentation enhances the TPC as well as antioxidant activity of the BS extract (Juan et al., 2010). However, isoflavones have direct free radical quenching ability, with daidzein and genistein being particularly effective (Shon et al., 2007; Cho et al., 2009; Cho et al., 2011). Moreover, Kim et al. (2008) reported that the cheonggukjang extract and its constituents, genistein and daidzein, exhibited significant antioxidant activity in vitro. In the present study, higher in vitro antioxidant activities were found shown in FBS in cheonggukjang than the UFBS of the four cultivars tested. This result indicates that enhanced antioxidant effects of cheonggukjang made with BS were caused by the increased amount of total polyphenol content and aglycone isoflavone than that of the unfermented BS. In addition, the increased malonyl glucoside concentration during fermentation may also contribute to enhance the antioxidant activity of FBS (cheonggukjang) compared to the UFBS. In our previous study, the radical scavenging activity was increased from 53.6% to 93.9% according to the total phenolic and isoflavone-aglycone (daidzein) contents during cheonggukjang fermentation with potential probiotic B. subtilis CS90 (Cho et al., 2011). Recently, we found that the stronger antioxidant activity of cheonggukjnag made with two wild cultivars of BS as well as brown soybeans might be related to the markedly higher TPCs and isoflavone-aglycones and -malonylgycosides achieved during fermentation (Hwang et al., 2013; Shin et al., 2014), which is consistent with the current study and with Kwak et al. (2007).
In conclusions, this study first documented the changes in the TPCs and in the contents of isoflavones during cheonggukjang fermentation made with BS cultivars with a potential probiotic B. subtilis CSY191. In the case of four BS cultivars, including, Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong, the TPCs and isoflavone-aglycone contents were markedly increased, while the isoflavone-glycosides were decreased according to the β-glycosidase activities. Importantly, the TPCs, total isoflavone contents, and antioxidant activities were higher in cheonggukjang made with BS of Cheongja#3 cultivar than those of the cheonggukjang made with Cheongja, Geomjeong#5, Ilpumgeomjeong cultivars at 48 h of fermentation. Therefore, it is supposed that high antioxidant activity of cheonggukjang made with BS might be related to the higher TPCs and isoflavone-aglycone contents achieved during fermentation with B. subtilis CSY191. Moreover, cheonggukjang extract made with hybrid BS seeds supposed to be used for the commercial production of functional foods in near future.