The rapid increase of the greenhouse gas carbon dioxide (CO2) in the atmosphere is generally thought to be a major factor in climate change. These changes are increasingly viewed as a threat to both the global economy and the natural environment [1-4]. This increase in CO2 is mainly attributed to human activities [5-10]. However, methane (bases: CH4 50%–70%, CO2 30%–40%) is one of reproducible biomass energy, which can obtain available energy sources, but also generate abundant greenhouse gas that goes against the purity of CH4 [10]. It is essential to find an ideal material to work out the austere problem. Usually, CO2 capture and storage is an efficient green technology used to reduce emissions by transporting and storing CO2 in deep geologic formations. The capture methods can be classified as post-combustion, pre-combustion, and air separation followed by oxyfuel combustion [7]. Among the currently available technologies, post-combustion is the most easily applied technology. It involves use of absorption, adsorption, cryogenic distillation, membranes, and gas hydrates.
Up to now, high-performance adsorbents have been widely used for CO2 capture. Conventional solid adsorbents include zeolites [11], activated carbon [12], mesoporous silica [13], metal oxide-based adsorbents (e.g., MgO and CaO) [14], and metal-organic frameworks (MOFs) [15]. Nevertheless, in each case, there are defects in the adsorption process. Zeolites and mesoporous silica have the drawback of poor CO2 capture performance. With zeolites, adsorption of moisture could affect the stability of the zeolite frameworks, and demands high regeneration temperature (>573 K). This results in huge energy consumption for CO2 desorption. Meanwhile, although the adsorption capacity for CO2 of activated carbon is high due to pore sizes ranging from micropores to macropores, its selectivity for gases is low. Metal oxide-based adsorbents in the form of dry chemical absorbents, exhibit very high energy consumption in the process of adsorption/desorption (>673 K), and high cost for regeneration. Moreover, the processes for synthesis of MOFs are very expensive and complex, although they do have a high capacity for CO2 capture. Furthermore, moisture-sensitivity limits their application in power plants [7].
In recent years, porous carbons have become desirable adsorbents for carbon dioxide capture due to their high specific surface area, well-developed pore structure, ease of regeneration, easily modifiable surfaces, and high CO2 adsorption capacity at ambient pressure. Such materials include activated carbon, activated carbon fibers, carbon nanofibers, graphite nanofibers (GNFs), mesoporous carbons, and graphite [16-22]. Among these, activated carbons and activated carbon fibers have been widely used in the field of CO2 adsorption.
As one of the most important carbon materials, GNFs have been applied for gas storage, electrodes, filler, and catalyst support. However, few researchers have studied carbon fiber materials as a carbon source that might be activated for gas adsorption. This is due to the specific surface area of GNFs being lower than for the other carbon materials, which limits its application. Meng et al. [23] studied the effect of heat treatment on CO2 adsorption of activated GNFs with a high specific surface area (567 m2/g) achieved by KOH treatment. Their results exhibited the best CO2 adsorption capacity so far (59.2 mg/g). These GNFs exhibited a narrow pore-size distribution with a uniform distribution of pores on the fiber surface, which are particularly good characteristics for the frequent adsorption and desorption of carbon dioxide [18-25]. In all these cases, it is the total microporosity of the materials that is a critical factor in determining their adsorption capability, and chemical activation with KOH is a significant means to produce such microporosity.
The methods used to synthesize activated carbon include physical activation, chemical activation, and catalytic activation. In particular, chemical activation is considered an efficient method for preparation of activated carbon. KOH is usually used as a chemical activation agent because it can be used to prepare activated carbon with extremely high surface area and narrow micropore distribution, along with a porous structure in which micropores are predominant.
In previous work, the effect of activation temperature was investigated, but the influence of the ratio of the carbon/activation agent, was not fully settled. Therefore, we investigated KOH-activation of carbon by chemical activation to enhance CO2 adsorption capacity. KOH-activated GNFs were treated with KOH at GNFs/KOH weight ratios ranging from 0 to 5. The CO2 adsorption capacity of GNFs was determined using CO2 adsorption isotherms at 298 K and 1 bar.
The GNFs (straight type, length: 25–30 µm; Nanomirae Co., Ltd., Seoul, Korea) were activated with KOH at GNFs/KOH weight ratios ranging from 0 to 5, at 900℃. For ease of discussion, the GNF sorbent variants will be referred to as CK-1, CK-2, CK-3, CK-4, and CK-5. To prepare the KOH-activated GNFs, the impregnation process was initiated by mixing 1 g of GNFs with 0–5 g of KOH, in 10 g of water. The mixture was stirred for 4 h at 60℃ and then dried for 24 h at 100℃. The treated carbon was placed into a quartz tube reactor, heated to 900℃ at a rate of 5℃/min in flowing N2 (200 mL/min), and kept at this temperature for 2 h before cooling to room temperature. Then the activated GNFs were obtained using 5 wt% HCl and hot distilled water. This solution was filtered and then dried at 100℃
A scanning electron microscope (SEM; Hitachi S-4200 Ltd., Tokyo, Japan) was used to examine the pristine samples, and the morphological changes due to activation of the treated samples. Nitrogen sorption isotherms were determined to characterize the textural properties of the carbon materials at 77 K. The specific surface area and total pore volume were determined using the Brunauer-Emmett-Teller method based on adsorption determined with a surface area analyzer (BEL Corp., Toyonaka, Japan). The micropore and mesopore volume were calculated using the Horvath-Kawazoe and Barrett-Joyner-Halenda equations. CO2 adsorption was measured at 298 K and 1 atm. Furthermore, the parameters of the Dubinin-Astakhov equation were obtained from CO2 adsorption.
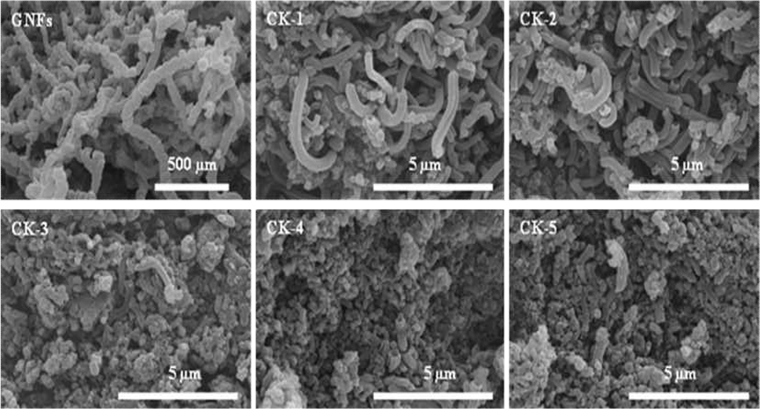
Fig. 1 shows images of the pristine and activated GNFs. From the images, one sees evident changes in the GNF length and surface morphology in the activated GNFs as the GNF/KOH weight ratio increases. Compared with GNFs, it can be noted that a high GNF/KOH weight ratio leads to an increased number of shorter GNFs (CK-1–CK-5) (Fig. 1).
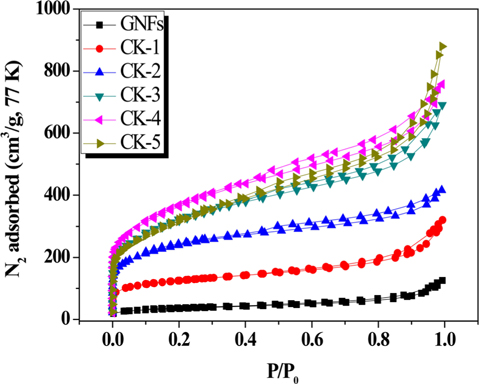
The effects of the GNF/KOH ratio on the pore structure of the GNFs were investigated using N2/77 K full isotherms to characterize their textural properties. The results are presented in Fig. 2, and International Union of Pure and Applied Chemistry (IUPAC) Type I and IV isotherm patterns can be seen. As shown in Fig. 2, when the relative pressure was less than 0.1, the rapid increase in the adsorption capacity of the prepared porous carbons indicates that there were many micropores; whereas a hysteresis loop at relative pressures above 0.3 suggests the occurrence of multilayer adsorption. This implies that both the treated and untreated GNFs have a broad pore-size distribution [23,24]. Comparing the pristine and treated GNFs, we conclude that KOH activation performed with GNF/KOH weight ratios from 1 to 5 contributes to the development of porosity.
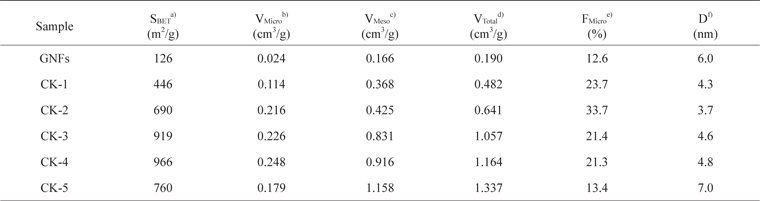
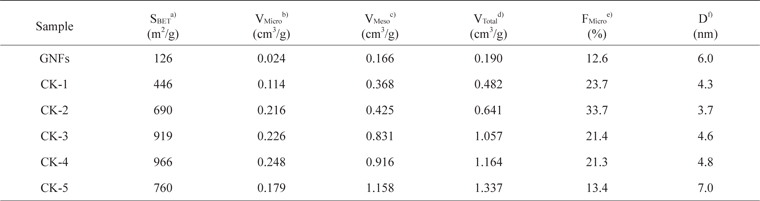
The specific surface area, total pore volume, mesopore volume, micropore volume, micropore fraction, and average pore diameter of the pristine and treated samples are summarized in Table 1. After KOH activation, the specific surface area, total pore volume, mesopore volume, and micropore volume of GNFs increased for weight ratios from 1 to 4 but decreased for sample CK-5. Metallic potassium as a reaction product will become metal vapor above 760℃, and the vaporized potassium can be removed from inside the graphite layers, which also leads to the formation of micropores [25]. Our results show that KOH activation of GNFs has an important effect on the increase of micropore volume. Among all the samples, the largest specific surface area and micropore volume were observed for sample CK-4.
Pore structure parameters for the nanoporous GNFs from the N2 adsorption isotherms as a function of the GNF/KOH ratio
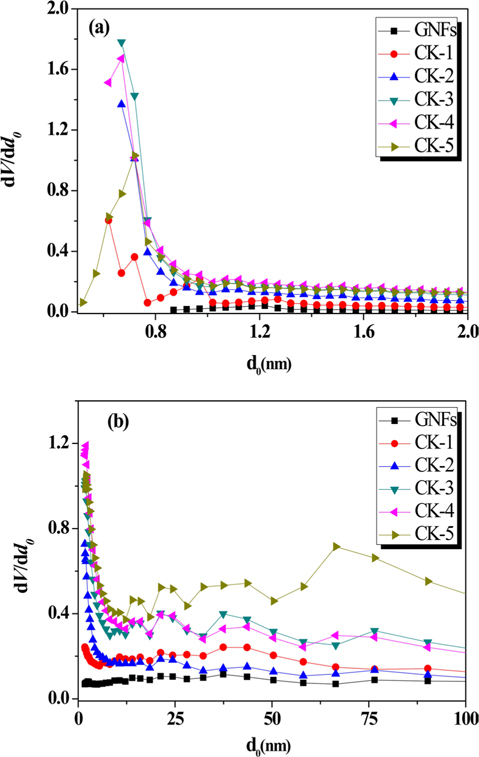
Fig. 3 represents the effect of carbon/agent ratio for pore size distributions of prepared activated carbons. Micropore size distributions and mesopore size distributions were calculated using the Horvath-Kawazoe method and Barrett-Joyner-Halenda method. These results show that they all possess a very narrow pore-size distribution, mainly concentrated in the range 1.4–2.0 nm. However, for all the activated GNFs, there is a broad peak with pore size 12.5–87.5 nm, which may be due to the collapse of the carbon framework at a high GNF/KOH ratio, resulting in the presence of large pores. Furthermore, as shown in Fig. 3b, it is interesting that the mesopore distribution of CK-4 is much broader than the others. This may be the reason for its CO2 adsorption capacity being a little less than for CK-3, though CK-4 had the largest micropore volume. Based on the above discussion, the increase of the GNF/KOH ration can promote the development of micropores [26-28].
In previous work with the carbon obtained after KOH activation, the activation mechanisms of different carbon sources, and effects of activation conditions, were investigated. In this study, the main activation mechanism of KOH-GNFs was similar to the mechanism of the KOH-activated carbon reaction, as follows [23]:
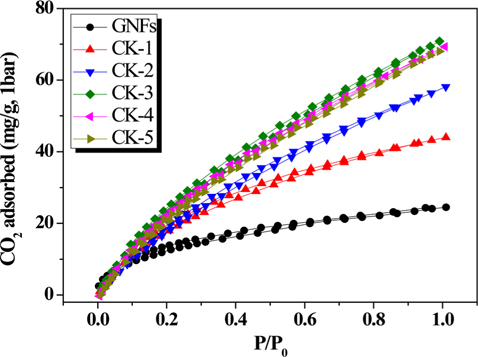
Up to now, it has been widely believed that the physical gas adsorption capacity of a sorbent material depends principally on a well-developed cellular structure and higher specific surface area [29]. In this paper, the activated carbon was synthesized using GNFs as carbon precursor and with KOH serving as an activation agent. These were used to estimate the effect of the carbonization temperature on the obtained carbon samples. Their CO2 adsorption performance was determined by CO2 adsorption measurement at 298 K and 1 bar. The test result is depicted in Fig. 4. From the graph in Fig. 4, one sees that the treated GNFs have a better CO2 adsorption capacity than the pristine GNFs. This shows that KOH activation increases the adsorption capacity of GNFs towards CO2. According to the CO2 adsorption isotherms, all the carbons showed high CO2 adsorption capacity in the order CK-3 > CK-4 > CK-5 > CK-2 > CK-1 > GNFs, which can be attributed to their high surface area and narrow micropore size distribution. Because the surface provides the adsorption sites and the pore volume is the volume for trapping CO2, the large number of adsorption sites and high trapping volume lead to high CO2 adsorption [10]. Furthermore, sample CK-3 exhibited the best CO2 adsorption capacity (70.8 mg/g). This result shows that the adsorption capacity of the treated GNFs was higher than the value reported by Jin et al. [30] (up to 52 mg/g using porous carbon nanotubes as a CO2 adsorbent) under similar conditions.
The prepared graphite nano fibers activated with KOH at several different weight ratios (GNF/KOH) at 900℃ were examined using SEM, N2/77 K full isotherms, and CO2 isothermal adsorption to characterize the morphology, textural properties, and CO2 adsorption capacity, respectively. From the results, it was found that the GNF/KOH ratio had a major influence on the CO2 adsorption capacity and textural properties of the GNFs. The KOH-activated GNFs were microporous and KOH activation increased their micro-porosity. The treated samples had improved CO2 adsorption capacity (up to 70.8 mg/g) as compared to non-activated GNFs.